The Multifaceted Role of LRRK2 in Parkinson's Disease
- PMID: 40309866
- PMCID: PMC12026217
- DOI: 10.3390/brainsci15040407
The Multifaceted Role of LRRK2 in Parkinson's Disease
Abstract
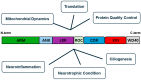
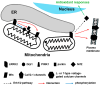
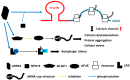
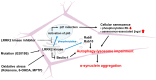
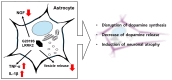
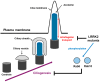
Leucine-rich repeat kinase 2 (LRRK2) is a multifunctional protein kinase intricately involved in the pathogeneses of various neurodegenerative diseases, particularly Parkinson's disease (PD). LRRK2 plays a pivotal role in mitochondrial function and cellular senescence by regulating key processes such as autophagy, oxidative stress, and protein aggregation. LRRK2 is also associated with ciliogenesis in regulating neuronal development. In addition, LRRK2 has been implicated as a putative mediator in neuroinflammation via promoting the reactivation of microglia and influencing cytokine production, a factor that may have therapeutic implications. Furthermore, mutations in LRRK2 have been found to impact the production of neurotrophic factors in astrocytes, the star-shaped glial cells of the central nervous system, thereby affecting neuronal health and contributing to the pathology of neurodegenerative diseases like PD. The multifaceted roles of LRRK2 in cellular senescence, interaction with LRS, neuroinflammation, the maintenance of mitochondria, and astrocyte function highlight its significance as a therapeutic target for neurodegenerative disorders.
Keywords: Parkinson’s disease; cellular senescence; ciliogenesis; leucine-rich repeat kinase 2; mitochondrial homeostasis; neuroinflammation; neurotrophic factor; translation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

