ATP-release pannexin channels are gated by lysophospholipids
- PMID: 40309905
- PMCID: PMC12045621
- DOI: 10.7554/eLife.107067
ATP-release pannexin channels are gated by lysophospholipids
Abstract
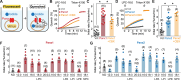
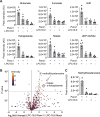
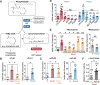
In addition to its role as cellular energy currency, adenosine triphosphate (ATP) serves as an extracellular messenger that mediates diverse cell-to-cell communication. Compelling evidence supports that ATP is released from cells through pannexins, a family of membrane proteins that form heptameric large-pore channels. However, the activation mechanisms that trigger ATP release by pannexins remain poorly understood. Here, we discover lysophospholipids as endogenous pannexin activators, using activity-guided fractionation of mouse tissue extracts combined with untargeted metabolomics and electrophysiology. We show that lysophospholipids directly and reversibly activate pannexins in the absence of other proteins. Secretomics experiments reveal that lysophospholipid-activated pannexin 1 leads to the release of not only ATP but also other signaling metabolites, such as 5'-methylthioadenosine, which is important for immunomodulation. We also demonstrate that lysophospholipids activate endogenous pannexin 1 in human monocytes, leading to the release of IL-1β through inflammasome activation. Our results provide a connection between lipid metabolism and purinergic signaling, both of which play major roles in immune responses.
Keywords: human; inflammasome; large pore channel; lipid signaling; molecular biophysics; secretomics; structural biology; untargeted metabolomics; xenopus.
© 2025, Henze et al.
Conflict of interest statement
EH, RB, BF, TS, EG, KM, LK, ML, JB, HL, TK No competing interests declared, FS FCS is a cofounder of Ascribe Bioscience and Holoclara Inc All other authors declare they have no competing interests
Figures




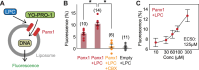



Update of
-
ATP-release pannexin channels are gated by lysophospholipids.bioRxiv [Preprint]. 2025 Feb 5:2023.10.23.563601. doi: 10.1101/2023.10.23.563601. bioRxiv. 2025. Update in: Elife. 2025 May 01;14:RP107067. doi: 10.7554/eLife.107067. PMID: 37961151 Free PMC article. Updated. Preprint.
References
-
- Alzola E, Pérez-Etxebarria A, Kabré E, Fogarty DJ, Métioui M, Chaïb N, Macarulla JM, Matute C, Dehaye JP, Marino A. Activation by P2X7 agonists of two phospholipases A2 (PLA2) in ductal cells of rat submandibular gland. The Journal of Biological Chemistry. 1998;273:30208–30217. doi: 10.1074/jbc.273.46.30208. - DOI - PubMed
-
- Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. The Journal of Biological Chemistry. 2010;285:24420–24431. doi: 10.1074/jbc.M110.115444. - DOI - PMC - PubMed
-
- Berchtold LA, Miani M, Diep TA, Madsen AN, Cigliola V, Colli M, Krivokapic JM, Pociot F, Eizirik DL, Meda P, Holst B, Billestrup N, Størling J. Pannexin-2-deficiency sensitizes pancreatic β-cells to cytokine-induced apoptosis in vitro and impairs glucose tolerance in vivo. Molecular and Cellular Endocrinology. 2017;448:108–121. doi: 10.1016/j.mce.2017.04.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

