Washout on Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions-A Review on Its Frequency and Possible Causes
- PMID: 40310346
- PMCID: PMC12025567
- DOI: 10.3390/diagnostics15080998
Washout on Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions-A Review on Its Frequency and Possible Causes
Abstract
In all imaging methods, including contrast-enhanced ultrasound (CEUS), enhancement in the late phase (LP) is an important criterion for differentiating between benign and malignant focal liver lesions (FLLs). In general, malignant liver lesions are characterized by hypoenhancement and washout in the LP. A lesion with LP hyperenhancement or isoenhancement in the non-cirrhotic liver is usually benign. However, LP hypoenhancement in benign lesions is not so rare, and is even normal and the standard for some lesions, and there are exceptions for each tumor entity that can represent a diagnostic challenge. Knowing these contrast patterns and exceptions is key for correct diagnosis and patient management. The following narrative review describes the contrast behaviors and the frequency of washout and LP hypoenhancement for common as well as rare benign liver lesions and analyzes its causes.
Keywords: benign liver lesions; contrast-enhanced ultrasound (CEUS); hypoenhancement; late phase (LP); washout.
Conflict of interest statement
The authors declare that they have no financial conflicts of interest with regard to the content of this report. Martin Krix is an employee of Bracco.
Figures

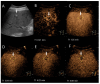
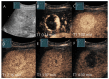
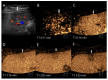
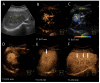
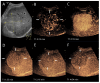
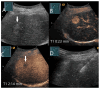
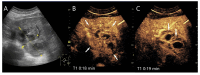
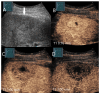
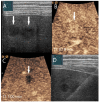
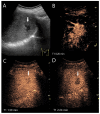
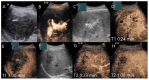
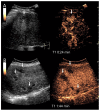
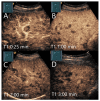
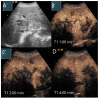
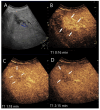
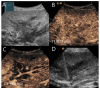
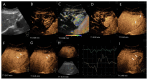
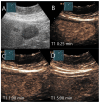
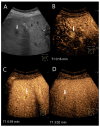
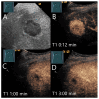
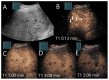
Similar articles
-
Contrast-enhanced ultrasound of hepatocarcinogenesis in liver cirrhosis.Chin Med J (Engl). 2012 Sep;125(17):3104-9. Chin Med J (Engl). 2012. PMID: 22932189
-
Imaging Features of Hepatocellular Carcinoma in the Non-Cirrhotic Liver with Sonazoid-Enhanced Contrast-Enhanced Ultrasound.Diagnostics (Basel). 2022 Sep 20;12(10):2272. doi: 10.3390/diagnostics12102272. Diagnostics (Basel). 2022. PMID: 36291962 Free PMC article.
-
Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS).Ultraschall Med. 2009 Aug;30(4):376-82. doi: 10.1055/s-0028-1109672. Epub 2009 Aug 17. Ultraschall Med. 2009. PMID: 19688669
-
Differentiating malignant from benign splenic lesions: a meta-analysis and pictorial review of imaging features.Abdom Radiol (NY). 2024 Aug;49(8):2833-2857. doi: 10.1007/s00261-024-04447-w. Epub 2024 Jun 20. Abdom Radiol (NY). 2024. PMID: 38900328 Review.
-
The current status of contrast-enhanced ultrasound in China.J Med Ultrason (2001). 2010 Jul;37(3):97-106. doi: 10.1007/s10396-010-0264-9. Epub 2010 Apr 21. J Med Ultrason (2001). 2010. PMID: 27278008 Review.
References
-
- Dietrich C.F., Nolsøe C.P., Barr R.G., Berzigotti A., Burns P.N., Cantisani V., Chammas M.C., Chaubal N., Choi B.I., Clevert D.-A., et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver–Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020;46:2579–2604. doi: 10.1016/j.ultrasmedbio.2020.04.030. - DOI - PubMed
-
- Lee J.Y., Minami Y., Choi B.I., Lee W.J., Chou Y.-H., Jeong W.K., Park M.-S., Kudo N., Lee M.W., Kamata K., et al. The AFSUMB Consensus Statements and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound using Sonazoid. Ultrasonography. 2020;39:191–220. doi: 10.14366/usg.20057. - DOI - PMC - PubMed
-
- Kong W., Wang W., Huang B., Ding H., Mao F. Value of wash-in and wash-out time in the diagnosis between hepatocellular carcinoma and other hepatic nodules with similar vascular pattern on contrast-enhanced ultrasound. J. Gastroenterol. Hepatol. 2014;29:576–580. doi: 10.1111/jgh.12394. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

