Next-generation rifamycins for the treatment of mycobacterial infections
- PMID: 40310456
- PMCID: PMC12067261
- DOI: 10.1073/pnas.2423842122
Next-generation rifamycins for the treatment of mycobacterial infections
Abstract
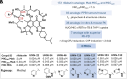
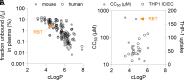
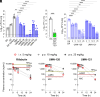
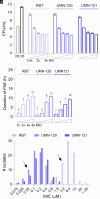
Mycobacterium abscessus is a rapidly growing nontuberculous Mycobacterium causing severe pulmonary infections, especially in immunocompromised individuals and patients with underlying lung conditions like cystic fibrosis (CF). While rifamycins are the pillar of tuberculosis treatment, their efficacy against M. abscessus lung disease is severely compromised by intrabacterial ADP-ribosylation. Additionally, rifamycins induce cytochrome P450 3A4 (CYP3A4), a major human drug-metabolizing enzyme, further limiting their use in patients with comorbidities that require treatment with CYP3A4 substrates such as CF and HIV coinfection. We chemically reengineered rifabutin to enhance its potency against M. abscessus by blocking intrabacterial inactivation and eliminate drug-drug interactions by removing induction of CYP3A4 gene expression. We have designed and profiled a series of C25-substituted derivatives resistant to intracellular inactivation and lacking CYP3A4 induction, while retaining excellent pharmacological properties. Against Mycobacterium tuberculosis, devoid of ADP-ribosyltransferase, the frontrunners are equipotent to rifabutin, suggesting superior clinical utility since they no longer come with the drug interaction liability typical of rifamycins. Prioritized compounds demonstrated superior antibacterial activity against a panel of M. abscessus clinical isolates, were highly bactericidal against replicating and drug-tolerant nonreplicating bacteria in caseum surrogate and were active against intracellular bacteria. As single agents, these rifamycins were as effective as a standard-of-care four-drug combination in a murine model of M. abscessus lung infection.
Keywords: Mycobacterium abscessus; drug discovery; preclinical development candidate; pulmonary infection; rifamycin.
Conflict of interest statement
Competing interests statement:PCT/US2023/034573 (16).
Figures

References
-
- Fox W., Ellard G. A., Mitchison D. A., Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int. J. Tuberc. Lung Dis. 3, S231–279 (1999). - PubMed
-
- Loeliger A., et al. , Protease inhibitor-containing antiretroviral treatment and tuberculosis: Can rifabutin fill the breach? Int. J. Tuberc Lung Dis. 16, 6–15 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

