Reirradiation for recurrent glioblastoma: the significance of the residual tumor volume
- PMID: 40310485
- PMCID: PMC12198277
- DOI: 10.1007/s11060-025-05042-9
Reirradiation for recurrent glioblastoma: the significance of the residual tumor volume
Abstract
Purpose: Recurrent glioblastoma has a poor prognosis, and its optimal management remains unclear. Reirradiation (re-RT) is a promising treatment option, but long-term outcomes and optimal patient selection criteria are not well established.
Methods: This study analyzed 71 patients with recurrent CNS WHO grade 4, IDHwt glioblastoma (GBM) who underwent re-RT at the University of Erlangen-Nuremberg between January 2009 and June 2019. Imaging follow-ups were conducted every 3 months. Progression-free survival (PFS) was defined using RANO criteria. Outcomes, feasibility, and toxicity of re-RT were evaluated. Contrast-enhancing tumor volume was measured using a deep learning auto-segmentation pipeline with expert validation and jointly evaluated with clinical and molecular-pathologic factors.
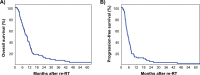
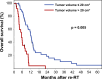
Results: Most patients were prescribed conventionally fractionated re-RT (84.5%) with 45 Gy in 1.8 Gy fractions, combined with temozolomide (TMZ, 49.3%) or lomustine (CCNU, 12.7%). Re-RT was completed as planned in 94.4% of patients. After a median follow-up of 73.8 months, 88.7% of patients had died. The median overall survival was 9.6 months, and the median progression-free survival was 5.3 months. Multivariate analysis identified residual contrast-enhancing tumor volume at re-RT (HR 1.040 per cm3, p < 0.001) as the single dominant predictor of overall survival.
Conclusion: Conventional fractionated re-RT is a feasible and effective treatment for recurrent high-grade glioma. The significant prognostic impact of residual tumor volume highlights the importance of combining maximum-safe resection with re-RT for improved outcomes.
Keywords: Chemoradiation; Glioblastoma; Prognostic factors; Radiotherapy; Recurrence; Reirradiation; Tumor volume.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors state that they have no conflict of interest.
Figures
References
-
- Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996 - PubMed
-
- Gorlia T et al (2012) New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC brain tumour group phase I and II clinical trials. Eur J Cancer 48(8):1176–1184 - PubMed
-
- Barker FG 2nd et al (1998) Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 42(4):709–720 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

