Enantiomeric Excess Bupivacaine in a Lavender Oil NLC Tested in a Melanoma Model: Prolonged Release and Anticancer Effect
- PMID: 40310503
- PMCID: PMC12135038
- DOI: 10.1021/acs.molpharmaceut.5c00254
Enantiomeric Excess Bupivacaine in a Lavender Oil NLC Tested in a Melanoma Model: Prolonged Release and Anticancer Effect
Abstract
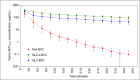
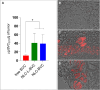
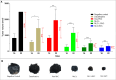
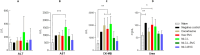
Recent studies have highlighted the potential of local anesthetics (LA) as adjuvants in cancer treatment, specifically by increasing survival rates when used in surgical excisions. However, the clinical use of LA is restricted due to their systemic toxicity. The development of drug delivery systems could address this issue and advance the utilization of these molecules. In this research, we explored the pharmacokinetics (using microdialysis probes) and antitumor properties of a nanostructured lipid carrier (NLC) formulation containing the commercially available enantiomeric excess form of bupivacaine (BVCS75). This NLC was prepared with lavender oil (NLC-L-BVC), an excipient with inherent antitumor properties. We compared this formulation to a control (NLC-BVC) using synthetic lipids. Pharmacokinetic assessments of the NLCs confirmed the sustained release of BVCS75 within the tumor, characterized by a reduced elimination rate constant and longer half-life (∼6×). The encapsulation of BVCS75 within nanoparticles (whether natural or synthetic) enhanced its effectiveness in treating the primary tumor, resulting in the inhibition of tumor growth (70% with NLC-L-BVC and 72% with NLC-BVC), outperforming free BVC (17% inhibition). However, the association of lavender oil with BVCS75 in an NLC did not yield synergistic properties. Furthermore, all BVCS75 treatments (whether free or encapsulated) improved animal survival rates. These findings confirm that encapsulation of bupivacaine in NLC can prolong drug action at the local site, contributing to improved local antitumor therapy while mitigating systemic effects.
Keywords: bupivacaine; drug delivery; lavender oil; melanoma; nanostructured lipid carriers.
Figures





Similar articles
-
Bupivacaine (S75:R25) Loaded in Nanostructured Lipid Carriers: Factorial Design, HPLC Quantification Method and Physicochemical Stability Study.Curr Drug Deliv. 2018;15(3):388-396. doi: 10.2174/1567201814666170726101113. Curr Drug Deliv. 2018. PMID: 28745230
-
Optimised NLC: a nanotechnological approach to improve the anaesthetic effect of bupivacaine.Int J Pharm. 2017 Aug 30;529(1-2):253-263. doi: 10.1016/j.ijpharm.2017.06.066. Epub 2017 Jun 24. Int J Pharm. 2017. PMID: 28655546
-
Local anesthetic effects of bupivacaine loaded lipid-polymer hybrid nanoparticles: In vitro and in vivo evaluation.Biomed Pharmacother. 2017 May;89:689-695. doi: 10.1016/j.biopha.2017.01.175. Epub 2017 Mar 6. Biomed Pharmacother. 2017. PMID: 28267672
-
Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the delivery of bioactives sourced from plants: part I - composition and production methods.Expert Opin Drug Deliv. 2024 Oct;21(10):1479-1490. doi: 10.1080/17425247.2024.2410951. Epub 2024 Oct 7. Expert Opin Drug Deliv. 2024. PMID: 39370828 Review.
-
Nanostructured lipid carriers for site-specific drug delivery.Biomed Pharmacother. 2018 Jul;103:598-613. doi: 10.1016/j.biopha.2018.04.055. Epub 2018 Apr 24. Biomed Pharmacother. 2018. PMID: 29677547 Review.
References
-
- de Moura L. D., Ribeiro L. N. M., de Carvalho F. V., Rodrigues da Silva G. H., Lima Fernandes P. C., Brunetto S. Q., Ramos C. D., Velloso L. A., de Araújo D. R., de Paula E.. Docetaxel and Lidocaine Co-Loaded (NlC-in-Hydrogel) Hybrid System Designed for the Treatment of Melanoma. Pharmaceutics. 2021;13(10):1552–1576. doi: 10.3390/pharmaceutics13101552. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

