Real-world effectiveness of darolutamide in metastatic castration-resistant prostate cancer
- PMID: 40310703
- PMCID: PMC12230269
- DOI: 10.1530/ERC-24-0188
Real-world effectiveness of darolutamide in metastatic castration-resistant prostate cancer
Abstract
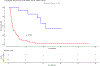
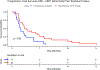
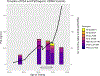
Darolutamide is a second-generation androgen receptor (AR) antagonist (2GARA) with established benefit in treating patients with non-metastatic castration-resistant prostate cancer (M0-CRPC) and metastatic castration-sensitive prostate cancer. Its real-world effectiveness in the treatment of patients with metastatic (M1) CRPC, including those who have progressed on CYP17 inhibitors (CYP17Is) or other 2GARAs (enzalutamide/apalutamide), is not well-described. We assessed the real-world effectiveness of darolutamide in a racially diverse cohort of 44 M1-CRPC and 11 M0-CRPC patients and collected data on baseline and emerging AR mutations in circulating tumor DNA (ctDNA) in these patients. The median progression-free survival (PFS) was 2.15 months for M1-CRPC and 21.16 months for M0-CRPC patients. In the M1-CRPC cohort, the median PFS was longer in those who had only received prior CYP17Is compared to 2GARA-resistant patients (2.43 vs 1.61 months; P = 0.03). Darolutamide suppressed serum PSA levels by >50% in 5/44 M1-CRPC patients (11.4%), all previously 2GARA-naïve. M1-CRPC patients resistant only to CYP17Is had improved mean best percent PSA changes from baseline compared to 2GARA-resistant patients (4.68 vs 42.34%; P = 0.047). PFS was not significantly different between African-American and non-African-American patients, or between patients with and without AR mutations at baseline. AR mutations emerging or increasing in allele fraction in ctDNA upon darolutamide treatment included H875Y, H100Q, D891H, T878A, L702H, L329W, N767Y and AR copy number gain. In summary, darolutamide may provide some benefit in CYP17I-refractory M1-CRPC patients, even in the presence of AR mutations. Resistance to other 2GARAs may significantly decrease benefit from darolutamide.
Keywords: M1-CRPC; darolutamide; health disparities; metastatic castration-resistant prostate cancer; next-generation sequencing.
Conflict of interest statement
Declaration of Interest
Patricia Castro declares significant financial interest in
Figures


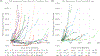

References
-
- ANNALA M, VANDEKERKHOVE G, KHALAF D, TAAVITSAINEN S, BEJA K, WARNER EW, SUNDERLAND K, KOLLMANNSBERGER C, EIGL BJ, FINCH D, OJA CD, VERGIDIS J, ZULFIQAR M, AZAD AA, NYKTER M, GLEAVE ME, WYATT AW & CHI KN 2018. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov, 8, 444–457. - PubMed
-
- ANTONARAKIS ES, ZHANG N, SAHA J, NEVALAITA L, IKONEN T, TSAI LJ, GARRATT C & FIZAZI K 2024. Prevalence and Spectrum of AR Ligand-Binding Domain Mutations Detected in Circulating-Tumor DNA Across Disease States in Men With Metastatic Castration-Resistant Prostate Cancer. JCO Precis Oncol, 8, e2300330. - PMC - PubMed
-
- ARMSTRONG AJ, SZMULEWITZ RZ, PETRYLAK DP, HOLZBEIERLEIN J, VILLERS A, AZAD A, ALCARAZ A, ALEKSEEV B, IGUCHI T, SHORE ND, ROSBROOK B, SUGG J, BARON B, CHEN L & STENZL A 2019. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. Journal of Clinical Oncology, 37, 2974–2986. - PMC - PubMed
-
- ATTARD G, BORRE M, GURNEY H, LORIOT Y, ANDRESEN-DANIIL C, KALLEDA R, PHAM T & TAPLIN ME 2018. Abiraterone Alone or in Combination With Enzalutamide in Metastatic Castration-Resistant Prostate Cancer With Rising Prostate-Specific Antigen During Enzalutamide Treatment. J Clin Oncol, 36, 2639–2646. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

