Organic Photocatalyst Utilizing Triplet Excited States for Highly Efficient Visible-Light-Driven Polymerizations
- PMID: 40310755
- PMCID: PMC12096438
- DOI: 10.1021/acs.accounts.4c00847
Organic Photocatalyst Utilizing Triplet Excited States for Highly Efficient Visible-Light-Driven Polymerizations
Abstract
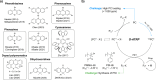
ConspectusUltraviolet (UV) light has traditionally been used to drive photochemical organic transformations, mainly due to the limited visible-light absorption of most organic molecules. However, the high energy associated with UV light often causes undesirable side reactions. In the late 2000s, MacMillan, Yoon, and Stephenson pioneered the use of visible light in conjunction with photocatalysts (PCs) to initiate organic transformations. This innovative approach overcame the limitations of UV light by utilizing visible-light-absorbing PCs in their photoexcited states for electron or energy transfer, generating reactive radical species and promoting the photoreactions. Furthermore, while the photocatalysis has predominantly relied on transition-metal complexes, concerns over the potential toxicity, cost, and sustainability of these metals have driven the development of organic PCs. These organic PCs eliminate the need for metal removal, offer structural diversity, and enable tuning of their properties, thus paving the way for the creation of a tailored library of PCs.In recent decades, significant advancements have been made in the development of novel organic PCs with diverse scaffolds, with a notable example being the work of Zhang et al. in 2016. They demonstrated that cyanoarene analogues, originally developed by Adachi et al. for thermally activated delayed fluorescence (TADF) in organic light-emitting diodes, could function effectively as PCs. Building on these insights, we developed a PC design platform featuring TADF compounds with twisted donor-acceptor structures, which paved the way for new PC discoveries. We showcased these PCs' ability (i) to generate long-lived lowest triplet excited (T1) states and (ii) to tune redox potentials by independently modifying donor and acceptor moieties. Through this platform, we discovered PCs with varying redox potentials and the capability to effectively populate T1 states, establishing structure-property relationships within our PC library and creating PC selection criteria for targeted reactions. Specifically, we tailored PCs for highly efficient reversible-deactivation radical polymerizations, including organocatalyzed atom transfer radical polymerization, photoinduced electron/energy transfer reversible addition-fragmentation chain transfer polymerization, and atom transfer radical polymerization with dual photoredox/copper catalysis as well as rapid free radical polymerizations. These advancements have also facilitated the development of functionalized, visible-light-cured adhesives for advanced display technologies. Furthermore, we investigated the origins of the exceptional catalytic performance of these PCs through comprehensive mechanistic studies, including electrochemical and photophysical measurements, quantum chemical calculations, and kinetics simulations. Specifically, we studied the formation and degradation of key PC intermediates in photocatalytic dehalogenations of alkyl and aryl halides. Our findings revealed a distinctive photodegradation pattern in the cyanoarene-based PCs, which significantly impact their catalytic efficiency in the reaction. Additionally, this discovery led us to introduce a concept of beneficial PC degradation for the first time.Over the past decades, organic photocatalysis based on the T1 state has become central to polymerization and organic synthesis. Utilizing our PC design platform, we have developed novel PCs and catalytic systems that enhance the overall efficiency of various organic transformations. In this overview of our contributions to visible-light-driven organic photocatalysis, we highlight the role of the T1 state in broadening applications through mechanistic analysis, enabling more sustainable transformations.
Figures










References
-
- Singh V. K., Yu C., Badgujar S., Kim Y., Kwon Y., Kim D., Lee J., Akhter T., Thangavel G., Park L. S., Lee J., Nandajan P. C., Wannemacher R., Milián-Medina B., Lüer L., Kim K. S., Gierschner J., Kwon M. S.. Highly Efficient Organic Photocatalysts Discovered via a Computer-Aided-Design Strategy for Visible-Light-Driven Atom Transfer Radical Polymerization. Nat. Catal. 2018;1:794–804. doi: 10.1038/s41929-018-0156-8. - DOI
-
- Lee Y., Kwon Y., Kim Y., Yu C., Feng S., Park J., Doh J., Wannemacher R., Koo B., Gierschner J., Kwon M. S.. A Water-Soluble Organic Photocatalyst Discovered for Highly Efficient Additive-Free Visible-Light-Driven Grafting of Polymers from Proteins at Ambient and Aqueous Environments. Adv. Mater. 2022;34:2108446. doi: 10.1002/adma.202108446. - DOI - PubMed
-
- Kwon Y., Lee J., Noh Y., Kim D., Lee Y., Yu C., Roldao J. C., Feng S., Gierschner J., Wannemacher R., Kwon M. S.. Formation and Degradation of Strongly Reducing Cyanoarene-Based Radical Anions towards Efficient Radical Anion-Mediated Photoredox Catalysis. Nat. Commun. 2023;14:92. doi: 10.1038/s41467-022-35774-5. - DOI - PMC - PubMed
-
- Kwon Y., Lee S., Kim J., Jun J., Jeon W., Park Y., Kim H. J., Gierschner J., Lee J., Kim Y., Kwon M. S.. Ultraviolet Light Blocking Optically Clear Adhesives for Foldable Displays via Highly Efficient Visible-Light Curing. Nat. Commun. 2024;15:2829. doi: 10.1038/s41467-024-47104-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

