Midtemperature CO2 Deoxygenation to CO over Oxygen Vacancies of Doped CeO2
- PMID: 40311100
- PMCID: PMC12086774
- DOI: 10.1021/acsami.4c17644
Midtemperature CO2 Deoxygenation to CO over Oxygen Vacancies of Doped CeO2
Abstract
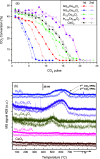
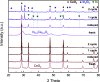
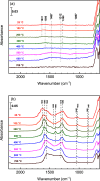
CO2 capture and utilization are a must for easing the global warming caused by the use of fossil fuels. Previous studies demonstrate the possibility of thermal deoxygenation of CO2 to CO over the vacancies of CeO2. This study examines the influence of the dopant to CeO2 on the deoxygenation of CO2 to CO, wherein the examined dopants include Zr, Gd, Sm, and In. Only In-doped CeO2 exhibits significant reactivity for CO2 deoxygenation in our sequential temperature-programmed reduction (TPR) and CO2-TPRx (temperature-programmed reaction) tests up to 700 °C. In0.5Ce0.5Oy after H2-TPR demonstrates a deoxygenation onset temperature as low as 400 °C and it can maintain a stable performance in cycle tests. X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) analyses indicate that In exsolutes from the fluorite framework during TPR and the exsoluted In0 become oxidized in the subsequent CO2 deoxygenation reaction. XPS indicates that the redox of Ce also occurs during TPR-CO2-TPRx with In0.5Ce0.5Oy. In2O3 by itself demonstrates a higher deoxygenation onset temperature, a lower per gram deoxygenation capacity, and a poorer stability than In0.5Ce0.5Oy under the same test conditions, while CeO2 is inactive. The results suggest a synergy between exsoluted In and the fluorite substrate, leading to the observed deoxygenation activity of In-doped CeO2.
Keywords: CO2; CeO2; deoxygenation; dopant; oxygen vacancy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Gao F.-Y.; Bao R.-C.; Gao M.-R.; Yu S.-H. Electrochemical CO2-to-CO conversion: electrocatalysts, electrolytes, and electrolyzers. J. Mater. Chem. A 2020, 8, 15458–15478. 10.1039/D0TA03525D. - DOI
-
- Scheffe J. R.; Steinfeld A. Thermodynamic Analysis of Cerium-Based Oxides for Solar Thermochemical Fuel Production. Energy Fuels 2012, 26, 1928–1936. 10.1021/ef201875v. - DOI
-
- Furler P.; Scheffe J. R.; Steinfeld A. Syngas production by simultaneous splitting of H2O and CO2via ceria redox reactions in a high-temperature solar reactor. Energy Environ. Sci. 2012, 5, 6098–6103. 10.1039/C1EE02620H. - DOI
LinkOut - more resources
Full Text Sources

