A phase II study of monalizumab and durvalumab in patients with recurrent/metastatic squamous cell carcinoma of the head and neck: results of the I2 cohort of the EORTC-HNCG-1559 trial (UPSTREAM)
- PMID: 40311183
- PMCID: PMC12084395
- DOI: 10.1016/j.esmoop.2025.104554
A phase II study of monalizumab and durvalumab in patients with recurrent/metastatic squamous cell carcinoma of the head and neck: results of the I2 cohort of the EORTC-HNCG-1559 trial (UPSTREAM)
Abstract
Background: Monalizumab (M), targeting the natural killer group 2A (NKG2A) receptor, has limited activity as monotherapy in recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN). Preliminary data of M and durvalumab (D) have shown encouraging activity in other tumor types.
Patients and methods: The UPSTREAM trial was an umbrella trial of targeted therapies and immunotherapy for R/M SCCHN. The immunotherapy 2 (I2) cohort was a phase II, randomized, open-label substudy evaluating the efficacy of D + M versus physician's choice (control). Patients non-eligible for the biomarker-driven cohorts and pretreated with PD(L)1, were included in the I2 cohort. The primary endpoint was the objective response rate (RECIST version 1.1) during the first 16 weeks.
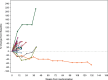
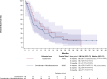
Results: Sixty-six patients with R/M SCCHN were included in the I2 cohort, of whom 60 were assessable (D + M: n = 42, control: n = 18): median age 62 years; 87% with two or three previous lines of treatment. In the D + M arm, one partial response (PR) was recorded, and stable disease (SD) was observed in 11 (26%). One PR was reported in the control arm and SD in 8 (44%). The median progression-free survival (PFS) was 2.0 and 3.1 months in the D + M arm and control arm, respectively. The median overall survival (OS) was 4.3 months (95% confidence interval 3.3-8.9 months) and 8.0 months (95% confidence interval 3.1-14.9 months) in the D + M and control arms, respectively. In the D + M arm, 4 (9%) patients reported grade ≥3 treatment-related adverse events.
Conclusion: The I2 substudy failed to demonstrate an activity of D + M in heavily pretreated patients with SCCHN previously exposed to anti-PD(L)1. No benefit was seen in PFS and OS.
Keywords: I2; UPSTREAM; immunotherapy; monalizumab; squamous cell carcinoma of the head and neck.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- World Health Organization International Agency for Research on Cancer. http://globocan.iarc.fr Available at.
-
- Pignon J.P., le Maître A., Maillard E., Bourhis J., MACH-NC Collaborative Group Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. - PubMed
-
- Burtness B., Harrington K.J., Greil R., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. - PubMed
-
- Vermorken J.B., Mesia R., Rivera F., et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

