The impact of extracellular matrix proteins on bovine fibro-adipogenic progenitor cell adhesion, proliferation, and differentiation in vitro
- PMID: 40312265
- PMCID: PMC12045701
- DOI: 10.14814/phy2.70283
The impact of extracellular matrix proteins on bovine fibro-adipogenic progenitor cell adhesion, proliferation, and differentiation in vitro
Abstract
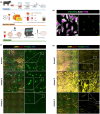
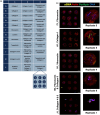
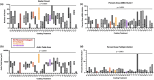
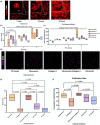
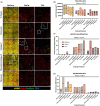
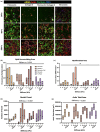
Fibro-adipogenic progenitor cells (FAPs) are mesenchymal stem cells that produce extracellular matrix (ECM) and intramuscular adipocytes in skeletal muscle. While FAPs have demonstrated responsiveness to their physical environment, there is limited knowledge of how the ECM substrate of FAPs impacts their differentiation, particularly in livestock animals. We hypothesized that the ECM substrate FAPs are cultured on will differentially impact their adherence, proliferation, and differentiation. Through an initial screen of 9 ECM proteins and their combinations, significant variation of bovine FAP attachment and differentiation across coatings was observed. The ECM substrates fibronectin, collagen 6, vitronectin, and a combination of fibronectin and collagen 6 were selected for further testing. Notably, fibronectin increased cell proliferation and attachment rates, without impairing FAP adipogenic or fibrogenic differentiation compared to the other coatings. Benefits of fibronectin were maintained at lower concentrations and when combined with less favorable coatings such as collagen 6. When assessed for their adipogenic potential on each coating at different substrate stiffnesses, lipid accumulation decreased with increasing substrate stiffness, while cell attachment increased on stiffer substrates. Overall, these results demonstrate the high responsiveness of FAPs to their ECM substrate, along with highlighting fibronectin as a preferred substrate for in vitro experiments with bovine FAPs.
Keywords: bovine; extracellular matrix; fibronectin; fibro‐adipogenic progenitor; mesenchymal stem cell.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no conflict of interest with the work presented.
Figures
Similar articles
-
The Role of Matrix Metalloproteinase-13 (MMP13) in TGFβ/BMP Pathway Regulation of Fibro-Adipogenic Progenitor (FAP) Differentiation.Cell Physiol Biochem. 2022 Dec 20;56(6):730-743. doi: 10.33594/000000596. Cell Physiol Biochem. 2022. PMID: 36537139
-
A single-cell atlas of bovine skeletal muscle reveals mechanisms regulating intramuscular adipogenesis and fibrogenesis.J Cachexia Sarcopenia Muscle. 2023 Oct;14(5):2152-2167. doi: 10.1002/jcsm.13292. Epub 2023 Jul 12. J Cachexia Sarcopenia Muscle. 2023. PMID: 37439037 Free PMC article.
-
Matrix stiffness and architecture drive fibro-adipogenic progenitors' activation into myofibroblasts.Sci Rep. 2022 Aug 9;12(1):13582. doi: 10.1038/s41598-022-17852-2. Sci Rep. 2022. PMID: 35945422 Free PMC article.
-
Thrown for a loop: fibro-adipogenic progenitors in skeletal muscle fibrosis.Am J Physiol Cell Physiol. 2023 Oct 1;325(4):C895-C906. doi: 10.1152/ajpcell.00245.2023. Epub 2023 Aug 21. Am J Physiol Cell Physiol. 2023. PMID: 37602412 Free PMC article. Review.
-
Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals.Animal. 2020 Feb;14(2):312-321. doi: 10.1017/S175173111900209X. Epub 2019 Oct 4. Animal. 2020. PMID: 31581971 Review.
References
-
- Agricultural Marketing Service Beef Grading Shields. Accessed May 14, 2024. https://www.ams.usda.gov/grades‐standards/beef/shields‐and‐marbling‐pict...
-
- Arredondo, R. , Poggioli, F. , Martínez‐Díaz, S. , Piera‐Trilla, M. , Torres‐Claramunt, R. , Tío, L. , & Monllau, J. C. (2021). Fibronectin‐coating enhances attachment and proliferation of mesenchymal stem cells on a polyurethane meniscal scaffold. Regenerative Therapy, 18, 480–486. 10.1016/j.reth.2021.11.001 - DOI - PMC - PubMed
-
- Bache, S. M. , & Wickham, H. (2014). magrittr: A Forward‐Pipe Operator for R. 2.0.3.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

