Brain iron accumulation in neurodegenerative disorders: Does air pollution play a role?
- PMID: 40312348
- PMCID: PMC12046710
- DOI: 10.1186/s12989-025-00622-z
Brain iron accumulation in neurodegenerative disorders: Does air pollution play a role?
Abstract
Background: Both excess brain Fe and air pollution (AP) exposures are associated with increased risk for multiple neurodegenerative disorders. Fe is a redox-active metal that is abundant in AP and even further elevated in U.S. subway systems. Exposures to AP and associated contaminants, such as Fe, are lifelong and could therefore contribute to elevated brain Fe observed in neurodegenerative diseases, particularly via nasal olfactory uptake of ultrafine particle AP. These studies tested the hypotheses that exogenously generated Fe oxide nanoparticles could reach the brain following inhalational exposures and produce neurotoxic effects consistent with neurodegenerative diseases and disorders in adult C57/Bl6J mice exposed by inhalation to Fe nanoparticles at a concentration similar to those found in underground subway systems (~ 150 µg/m3) for 20 days. Olfactory bulb sections and exposure chamber TEM grids were analyzed for Fe speciation. Measures included brain volumetric and diffusivity changes; levels of striatal and cerebellar neurotransmitters and trans-sulfuration markers; quantification of frontal cortical and hippocampal Aβ42, total tau, and phosphorylated tau; and behavioral alterations in locomotor activity and memory.
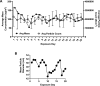
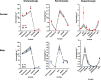
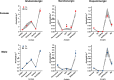
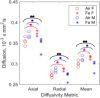
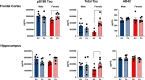
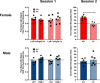
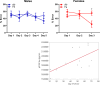
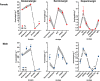
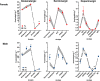
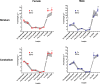
Results: Particle speciation confirmed similarity of Fe oxides (mostly magnetite) found on chamber TEM grids and in olfactory bulb. Alzheimer's disease (AD) like characteristics were seen in Fe-exposed females including increased olfactory bulb diffusivity, impaired memory, and increased accumulation of total and phosphorylated tau, with total hippocampal tau levels significantly correlated with increased errors in the radial arm maze. Fe-exposed males showed increased volume of the substantia nigra pars compacta, a region critical to the motor impairments seen in Parkinson's disease (PD), in conjunction with reduced volume of the trigeminal nerve and optic tract and chiasm.
Conclusions: Inhaled Fe oxide nanoparticles appeared to lead to olfactory bulb uptake. Further, these exposures reproduced characteristic features of neurodegenerative diseases in a sex-dependent manner, with females evidencing features similar to those seen in AD and effects in regions in males associated with PD. As such, prolonged inhaled Fe exposure via AP should be considered as a source of elevated brain Fe with aging, and as a risk factor for neurodegenerative diseases. The bases for dichotomous sex effects of inhaled Fe nanoparticles is as of yet unclear. Also as of yet unknown is how duration of such Fe exposures affect outcome, and/or whether exposures to inhaled Fe during early brain development enhances vulnerability to subsequent Fe exposures. Collectively, these findings suggest that regulation of air Fe levels, particularly in enclosed areas like subway stations, may have broad public health protective effects.
Keywords: Air pollution; Alzheimer’s disease; Iron; Memory; Olfactory bulb; Parkinson’s disease; Substantia nigra; Tau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participation: This study was carried out in accordance with relevant guidelines and regulations. All mice used were treated according to protocols approved by the University of Rochester Medical Center Institutional Animal Care and Use Committee and Committee on Animal Resources (approval #102208/2010-046E) and in accordance with NIH guidelines. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Baringer SL, Simpson IA, Connor JR. Brain iron acquisition: an overview of homeostatic regulation and disease dysregulation. J Neurochem. 2023. 10.1111/jnc.15819. - PubMed
-
- Benarroch EE. Brain iron homeostasis and neurodegenerative disease. Neurology. 2009;72(16):1436. 10.1212/WNL.0b013e3181a26b30. - PubMed
-
- Tao Y, Wang Y, Rogers JT, Wang F. Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(2):679–90. 10.3233/JAD-140396. - PubMed
-
- Connor JR, Menzies SL, St Martin SM, Mufson EJ. A histochemical study of iron, transferrin, and ferritin in Alzheimer’s diseased brains. J Neurosci Res. 1992;31(1):75–83. 10.1002/jnr.490310111. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

