Field evaluation of the Bioline Malaria Ag P.f/Pan rapid diagnostic test: causes of microscopy discordance and performance in Uganda
- PMID: 40312382
- PMCID: PMC12044711
- DOI: 10.1186/s12936-025-05379-6
Field evaluation of the Bioline Malaria Ag P.f/Pan rapid diagnostic test: causes of microscopy discordance and performance in Uganda
Abstract
Background: Histidine Rich Protein 2 (HRP2)/pan-Lactate Dehydrogenase (pLDH) combination rapid diagnostic tests (RDTs) may address the shortcomings of RDTs that detect HRP2 alone. However, the relative contribution of the possible causes of discordant results (RDT-negative and microscopy-positive) and performance in field settings across Uganda are poorly quantified.
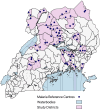
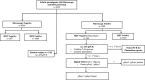
Methods: This study utilized samples from two cross-sectional surveys conducted in 32 districts at 64 sites across Uganda between November 2021 and March 2023 that enrolled 6354 febrile participants ≥ two years of age. Discordant samples (negative by HRP2/pLDH RDT and positive by microscopy) underwent quantitative PCR (qPCR) to detect and quantify parasitaemia. Those confirmed to be positive for Plasmodium falciparum at > 1 parasites/microlitre (p/µL) were tested for pfhrp2 and pfhrp3 deletions using digital PCR. Those that were negative or had P. falciparum detected at ≤ 1 p/µL underwent Plasmodium species testing using nested PCR. The performance of the Bioline Malaria Ag P.f/Pan combination RDT was evaluated by comparison with microscopy and qPCR.
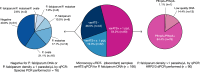
Results: There were 166 (8.4%) discordant samples out of 1988 microscopy positive samples. Of these, 90/166 (54.2%) were confirmed to contain P. falciparum at levels > 1 p/µL, whereas 76/166 (45.8%) were negative or had P. falciparum levels ≤ 1 p/µL. Only one P. falciparum positive sample was confirmed to have a deletion in pfhrp3. The primary reasons for RDT-negative, microscopy-positive discordance in samples testing negative for P. falciparum by PCR were non-falciparum species (37/76, 48.7%) or false positives by microscopy (31/76, 40.8%). The sensitivity of the Bioline Malaria Ag P.f/Pan combination RDT was high (> 91%) using either microscopy or qPCR as the gold standard. However, specificity was low (56.7%) when microscopy was used as the gold standard; it improved to 64.0% when qPCR was used as the gold standard.
Conclusion: The Bioline Malaria Ag P.f/Pan combination RDT was found to be highly sensitive in Uganda and reliable for ruling out malaria. False negative RDT results were primarily due to low density P. falciparum infections, non-falciparum infections, or incorrect microscopy results. In contrast, false positive RDT results were common, most likely due to persistent HRP2 antigenaemia in this high transmission setting though causes of false positive RDTs were not investigated. The low specificity of HRP2-based RDTs may result in overuse of anti-malarial drugs and missed diagnoses of non-malarial febrile illnesses.
Keywords: Plasmodium falciparum; Discordance; HRP2/pLDH combination rapid diagnostic test; Malaria; Performance; Pfhrp2; Pfhrp3; Sensitivity; Specificity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Makerere University School of Medicine Research and Ethics committee (2020–193), the Uganda National Council of Science and Technology (HS1097ES), University of California, San Francisco, Committee for Human Research (20–31769) and the London School of Hygiene and Tropical Medicine Ethics Committee (22615). Written informed consents (with assent from minors) were obtained from all study participants before enrollment. Competing interests: The authors declare no competing interests.
Figures
Update of
-
Field evaluation of the Bioline Malaria Ag P.f/Pan Rapid Diagnostic Test: Causes of Microscopy Discordance and Performance in Uganda.Res Sq [Preprint]. 2025 Feb 19:rs.3.rs-5629938. doi: 10.21203/rs.3.rs-5629938/v1. Res Sq. 2025. Update in: Malar J. 2025 May 1;24(1):138. doi: 10.1186/s12936-025-05379-6. PMID: 40034450 Free PMC article. Updated. Preprint.
Similar articles
-
Field evaluation of the Bioline Malaria Ag P.f/Pan Rapid Diagnostic Test: Causes of Microscopy Discordance and Performance in Uganda.Res Sq [Preprint]. 2025 Feb 19:rs.3.rs-5629938. doi: 10.21203/rs.3.rs-5629938/v1. Res Sq. 2025. Update in: Malar J. 2025 May 1;24(1):138. doi: 10.1186/s12936-025-05379-6. PMID: 40034450 Free PMC article. Updated. Preprint.
-
False-negative malaria rapid diagnostic tests in Rwanda: impact of Plasmodium falciparum isolates lacking hrp2 and declining malaria transmission.Malar J. 2017 Mar 20;16(1):123. doi: 10.1186/s12936-017-1768-1. Malar J. 2017. PMID: 28320390 Free PMC article.
-
Community-based surveys for Plasmodium falciparum pfhrp2 and pfhrp3 gene deletions in selected regions of mainland Tanzania.Malar J. 2020 Nov 4;19(1):391. doi: 10.1186/s12936-020-03459-3. Malar J. 2020. PMID: 33148255 Free PMC article.
-
Summary of discordant results between rapid diagnosis tests, microscopy, and polymerase chain reaction for detecting Plasmodium mixed infection: a systematic review and meta-analysis.Sci Rep. 2020 Jul 29;10(1):12765. doi: 10.1038/s41598-020-69647-y. Sci Rep. 2020. PMID: 32728145 Free PMC article.
-
Force Protection Risks in AFRICOM, INDOPACOM and SOUTHCOM Due to Rapid Diagnostic Test Failures for Falciparum Malaria, 2016-2022.MSMR. 2023 Oct 1;30(10):7-11. MSMR. 2023. PMID: 37963222 Review.
References
-
- WHO. World malaria report 2023. Geneva: World Health Organization; 2023.
-
- National Malaria Control Division, Uganda Bureau of Statistics, ICF. Malaria Indicator Survey 2018–2019. Kampala, Uganda and Rockville, Maryland, USA. 2020.
-
- National Malaria Control Division, Ministry of Health. The Uganda Malaria Reduction Strategic Plan 2014–2020. Kampala, Uganda. 2014.
-
- WHO. Guidelines for malaria, 2023. Geneva: World Health Organization; 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

