Neonatal hypoxia leads to impaired intestinal function and changes in the composition and metabolism of its microbiota
- PMID: 40312410
- PMCID: PMC12045951
- DOI: 10.1038/s41598-025-00041-2
Neonatal hypoxia leads to impaired intestinal function and changes in the composition and metabolism of its microbiota
Abstract
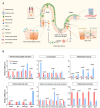
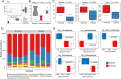
Neonatal hypoxia, a prevalent complication during the perinatal period, poses a serious threat to the health of newborns. The intestine, as one of the most metabolically active organs under stress conditions, is particularly vulnerable and susceptible to hypoxic injury. Using a neonatal hypoxic mouse model, we systematically investigated hypoxia-induced intestinal barrier damage and underlying mechanisms. Hypoxia caused significant structural abnormalities in the ileum and distal colon of neonatal mice, including increased numbers of F4/80+ cells (p = 0.0031), swollen mucus particles (p = 0.0119), and disrupted tight junction. At the genetic level, hypoxia caused dysregulation of the expression of genes involved in intestinal barrier function, including antimicrobial activity, immune response, intestinal motility, and nutrient absorption. Further 16 S rDNA sequencing revealed hypoxia-driven gut microbiota dysbiosis with general reduced microbial abundance and diversity (Chao1 = 0.1143, Shannon = 0.0571, and Simpson = 0.3429). Structural dysbiosis of the gut microbiota consequently perturbed metabolic homeostasis, especially enhancing the activity of glycolipid metabolism. Notably, results showed that hypoxia may interfere with neurotransmitter metabolism, thereby increasing the risk of neurological disorders.
Keywords: Gut microbiota; Intestinal barrier; Intestinal dysfunction; Mice; Neonatal hypoxia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

