Modeling the impact of vaccine campaigns on the epidemic transmission dynamics of chikungunya virus outbreaks
- PMID: 40312589
- PMCID: PMC12283388
- DOI: 10.1038/s41591-025-03684-w
Modeling the impact of vaccine campaigns on the epidemic transmission dynamics of chikungunya virus outbreaks
Abstract
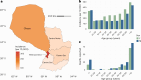
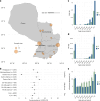
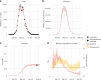
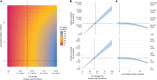
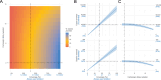
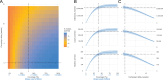
A licensed chikungunya vaccine now exists; however, it remains unclear whether it could be deployed during outbreaks to reduce the health burden. We used an epidemic in Paraguay as a case study. We conducted a seroprevalence study and used models to reconstruct epidemic transmission dynamics, providing a framework to assess the theoretical impact of a vaccine had it been available. We estimated that 33.0% (95% confidence interval (CI) 30.1-36.0%) of the population became infected during the outbreak. Of these individuals, 6.3% (95% CI 5.8-6.9%) were detected by the surveillance system, with a mean infection fatality ratio of 0.013% (95% CI 0.012-0.014%). A disease-blocking vaccine with 75% efficacy deployed in 40% of individuals aged ≥12 years over a 3-month period would have prevented 34,200 (95% CI 30,900-38,000) cases, representing 23% of all cases, and 73 (95% CI 66-81) deaths. If the vaccine also leads to infection blocking, 88% of cases would have been averted. These findings suggest that the vaccine is an important new tool to control outbreaks.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.R.d.S. and H.S. are paid consultants for Valneva in support of their Phase IV studies. H.S. has also received consultancy support from the Gavi Alliance. The other authors declare no competing interests.
Figures

References
-
- Weaver, S. C. & Forrester, N. L. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res.120, 32–39 (2015). - PubMed
-
- Kang, H. et al. Chikungunya seroprevalence, force of infection, and prevalence of chronic disability after infection in endemic and epidemic settings: a systematic review, meta-analysis, and modelling study. Lancet Infect. Dis.24, 488–503 (2024). - PubMed
-
- Salje, H. & Cortés Azuero, O. The deadly potential of chikungunya virus. Lancet Infect. Dis.24, 442–444 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

