Supplemental nucleus pulposus allograft in patients with lumbar discogenic pain: results of a prospective feasibility study
- PMID: 40312677
- PMCID: PMC12044970
- DOI: 10.1186/s12891-025-08701-0
Supplemental nucleus pulposus allograft in patients with lumbar discogenic pain: results of a prospective feasibility study
Abstract
Background: Degeneration of the intervertebral disc is a significant source of chronic axial low back pain. Direct supplementation of degenerated nucleus pulposis (NP) tissue with intradiscally delivered allogeneic NP represents an opportunity to bridge the treatment gap between failed conservative care and spine surgery for patients with lumbar discogenic pain.
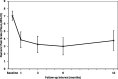
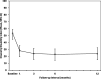
Methods: Prospective, single-arm clinical study conducted at 6 sites in the US. The primary objective was to determine the magnitude of improvement in back pain severity and back function in patients with chronic lumbar discogenic pain at 12 months after a single intradiscal supplementation procedure using a commercially available NP allograft at up to two vertebral levels identified on magnetic resonance imaging. Back pain severity was evaluated using an 11-point numeric rating scale (NRS) and back function using the Oswestry Disability Index (ODI). Minimal clinically important difference (MCID) and substantial clinical benefit (SCB) were set at ≥ 30% and ≥ 50% over baseline, respectively. The patient acceptable symptom state (PASS) threshold for pain severity was ≤ 3.
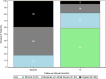
Results: Twenty-eight participants with a mean age of 44 ± 13 yrs. were enrolled and 22 provided 12-month outcomes. The average overall improvement in back pain severity was 43% through 12 months (p < 0.001). Approximately 64% (14 of 22) achieved the MCID in back pain at 12 months, with 55% (12 of 22) realizing SCB. Almost 60% (13 of 22) reported a 12-month back pain severity score of ≤ 3. The corresponding average decrease in ODI values was 50% (p < 0.001) with approximately 59% (13 of 22) of study participants achieving the MCID. At baseline approximately 82% (23 of 28) of participants reported severe or crippled back impairment compared to 18% (4 of 22) at 12 months (p < 0.001).
Conclusion: The results of this study provide additional evidence that supplementation of the degenerated intervertebral disc with intradiscally delivered allogeneic NP is associated with clinically significant pain palliation and functional improvement.
Trial registration: This trial was prospectively registered at ClinicalTrials.gov on December 30, 2021 (NCT05201287).
Keywords: Allograft; Back pain; Degenerative disc disease; Discogenic; Intradiscal; Nucleus pulposus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All patients provided informed consent. The study was reviewed and approved by an independent institutional review board (IRB), Sterling IRB (Atlanta, GA, USA). The trial was conducted in accordance with the Declaration of Helsinki and prospectively registered at ClinicalTrials.gov on December 30, 2021 (NCT05201287). Consent for publication: Not applicable. Competing interests: DPB is a scientific advisor to Vivex Biologics; received grants or contracts from Medtronic, Medical Metrics, Avanos, Relievant, Boston Scientific, Stryker, Sollis Pharmaceuticals, Simplify Medical, Lenoss Medical, Spine BioPharma, Eliem Therapeutics, Smart Soft, Tissue Tech, Vivex, Stratus Medical, Restorative Therapies, Kolon, TissueGene, Companion Spine, DiscGenics; royalties from VIVEX and IZI; consulting fees from Medtronic, Spineology, Merit Medical, Johnson & Johnson, IZI, Techlamed, Peterson Enterprises, Medical Metrics, Avanos, Boston Scientific, Sollis Pharmaceuticals, Simplify Medical, Stryker, Lenoss Medical, Spine BioPharma, Piramal, ReGelTec, Nanofuse, Spinal Simplicity, Pain Theory, Spark Biomedical, Micron Medical Corp, Bronx Medical, Smart Soft, Tissue Tech, RayShield, Stayble, Thermaquil, Vivex, Stratus Medical, Genesys, Abbott, Eliquence, SetBone Medical, Amber Implants, Cerapedics, Neurovasis, Varian Medical Systems, Companion Spine, DiscGenics, Discure, SpinaFX, PainTEQ; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Artio, Sophiris, Eleven Biotherapeutics, Flow Forward, Lenoss Medical, ReGelTech, Spark Biomedical; and support for attending meetings and/or travel from Medtronic, ReGelTec, Nanofuse, Talosix, Spinal Simplicity, Pain Theory, Spark Biomedical, Smart Soft, Tissue Tech, Bronx Medical, Thermaquil, Vivex, Genesys, SetBone Medical, Amber Implants, Cerapedics, SpinaFX. TTD received consulting fees from Abbott, Boston Scientific, Biotronik; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott, Boston Scientific, Biotronik. RKN received consulting fees from Vivex, Boston Scientific, Ferring Pharmaceuticals. MJD is a scientific advisor to Vivex Biologics; received grants or contracts from Spine BioPharma, Restorative, Novartis, SPR, Saol, Paradigm; royalties from Springer; patents from iSpine Ingenuity. SC received grants or contracts from the Cleveland Clinic. ESY received consulting fees from Neurovasis. JWF received support for attending meetings and/or travel from Medtronic, Stryker, Nevro, Seattle Science Foundation, HMP Global, American Society of Neuroradiology, American Society of Spine Radiology; received stock or stock options from BackTable LLC. JEB received support for medical writing from Vivex Biologics; consulting fees from Vivex. NM received consulting fees from Vivex Biologics. The other author, KA, reports no additional conflicts of interest.
Figures
References
-
- Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337–56. - PubMed
-
- Hickey DS, Hukins DW: Relation between the structure of the annulus fibrosus and the function and failure of the intervertebral disc. Spine (Phila Pa 1976). 1980, 5:106–116. - PubMed
-
- Johnson WE, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658–64. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

