A comparative study on efficacy and safety of modified partial stapled hemorrhoidopexy versus conventional hemorrhoidectomy: a prospective randomized controlled trial
- PMID: 40313128
- PMCID: PMC12046410
- DOI: 10.3393/ac.2024.00535.0076
A comparative study on efficacy and safety of modified partial stapled hemorrhoidopexy versus conventional hemorrhoidectomy: a prospective randomized controlled trial
Abstract
Purpose: The long-term outcomes and efficacy of partial stapled hemorrhoidopexy (PSH) compared with those of conventional hemorrhoidectomy (CH) are not fully understood. This study aimed to introduce a modified PSH (mPSH) and compare its clinical efficacy and safety with those of CH.
Methods: A prospective randomized controlled trial was conducted. This study was performed at a single hospital and involved 6 colorectal surgeons. In total, 110 patients were enrolled between July 2019 and September 2020. Patients were randomly assigned to undergo either mPSH group (n=55) or CH group (n=55). The primary outcome was to compare postoperative average pain and postoperative peak pain using visual analog scale score between the 2 groups.
Results: The required duration of analgesia was shorter in the mPSH group than in the CH group, although the difference was not statistically significant (P=0.096). However, the laxative requirement duration (P<0.010), return to work (P<0.010), satisfaction score (P<0.010), and Vaizey score (P=0.014) were significantly better in the mPSH group. The average and peak postoperative pain scores were significantly lower in the mPSH group during the 15 days after surgery (P<0.001). The overall complication rate in both groups was 9.1%, with no significant difference between the groups (P=0.867).
Conclusion: The mPSH group demonstrated better improvement in symptoms, lower pain scores, and greater patient early satisfaction after surgery than the CH group. Therefore, this surgical technique appears to be a safe and effective alternative for CH.
Keywords: Hemorrhoids; Partial stapled hemorrhoidopexy; Prospective randomized controlled trial; Quality of life.
Conflict of interest statement
Chul Seung Lee is an editorial board member of this journal, but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
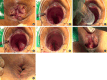
Figures


References
-
- Morinaga K, Hasuda K, Ikeda T. A novel therapy for internal hemorrhoids: ligation of the hemorrhoidal artery with a newly devised instrument (Moricorn) in conjunction with a Doppler flowmeter. Am J Gastroenterol. 1995;90:610–3. - PubMed
LinkOut - more resources
Full Text Sources

