Berberine-Functionalized Bismuth-Doped Carbon Dots in a Pathogen-Responsive Hydrogel System: A Multifaceted Approach to Combating Periodontal Diseases
- PMID: 40313185
- PMCID: PMC12080333
- DOI: 10.1021/acsnano.5c00561
Berberine-Functionalized Bismuth-Doped Carbon Dots in a Pathogen-Responsive Hydrogel System: A Multifaceted Approach to Combating Periodontal Diseases
Abstract
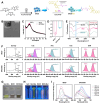
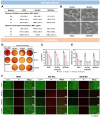
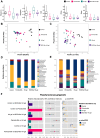
Periodontal disease, a global health burden linked to dysbiotic oral polymicrobial communities and disrupted immune-inflammatory responses, is critically mediated byPorphyromonas gingivalis(Pg)─the keystone pathogen that sabotages host immunity, triggers tissue inflammation and destruction, and disrupts microbiota balance. Effective therapies should combine antimicrobial action, immune modulation, virulence suppression, and microbiome restoration. Bismuth ions and berberine, which exhibit antimicrobial and epithelial barrier-protecting effects, show potential effectiveness in treating periodontal diseases but face practical limitations due to poor water solubility and bioavailability. To address this, we developed bismuth-doped carbon dots functionalized with structure-modified berberine (BiCD-Ber) as a multifunctional nanomedicine. BiCD-Ber eradicated Pg in various forms, restored Pg-perturbed immune responses in gingival fibroblasts, and preserved epithelial barrier integrity. The doped bismuth ions neutralized Pg virulence factors by blocking the catalytic sites of gingipains. To facilitate in vivo delivery, BiCD-Ber was encapsulated in a disulfide-modified hyaluronic acid hydrogel that degrades in response to Pg metabolites. This BiCD-Ber hydrogel system modulated subgingival microbiota, alleviated inflammation in gingiva, and thereby prevented alveolar bone loss. This approach to concurrently eliminating Pg, modulating inflammatory responses , suppressing virulence factors, and restoring microbiota showcases great potential in managing periodontitis effectively.
Keywords: alveolar bone loss; immune-responses modulation; pathogen-responsive hydrogel; periodontitis; subgingival microbiota.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

