Living hybrid material based on probiotic with photothermal properties inhibits PD-L1 expression after tumouricidal photothermal therapy
- PMID: 40313568
- PMCID: PMC12041808
- DOI: 10.12336/biomatertransl.2025.01.006
Living hybrid material based on probiotic with photothermal properties inhibits PD-L1 expression after tumouricidal photothermal therapy
Abstract
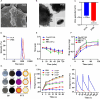
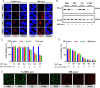
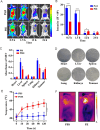
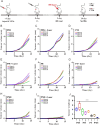
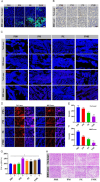
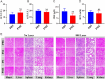
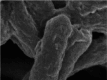
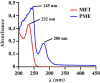
Photothermal therapy is a safe and effective tumour treatment strategy due to its excellent spatiotemporal controllability. However, interferon gamma in the tumour microenvironment is upregulated after photothermal therapy, which enhances the expression of programmed cell death ligand 1 (PD-L1) in tumour cells. This further promotes immunosuppression and tumour metastasis, resulting in a poor prognosis in cancer therapy. Traditional nanodrugs often face challenges in penetrating the dense extracellular matrix of solid tumours, whereas certain probiotics possess the ability to specifically colonise the core regions of tumours. In this research, we used Escherichia coli Nissle 1917 (ECN) as a chassis cell and self-assembly polydopamine (PDA) on the ECN surface. The black PDA@ECN (notes as PE) actively colonises at the tumour site and produces a photothermal effect under 808 nm laser irradiation to kill tumour cells. To overcome the high expression of PD-L1 induced after photothermal therapy, metformin (MET) was also encapsulated in PE to form PDA@MET@ECN (notes as PME). In vivo experiments demonstrated that PME effectively inhibited the PD-L1 expression and growth of CT26 tumour cells. Overall, PME reverses the immunosuppressive tumour microenvironment and enhances the effect of photothermal/immune therapy in tumour treatment.
Keywords: Escherichia coli Nissle 1917; biomaterials; photothermal therapy; programmed cell death ligand 1.
Conflict of interest statement
Conflicts of interest statement: The authors declare no conflicts of interest.
Figures


Similar articles
-
Functionalized biomimetic nanoparticles combining programmed death-1/programmed death-ligand 1 blockade with photothermal ablation for enhanced colorectal cancer immunotherapy.Acta Biomater. 2023 Feb;157:451-466. doi: 10.1016/j.actbio.2022.11.043. Epub 2022 Nov 25. Acta Biomater. 2023. PMID: 36442821
-
Towards Understanding Tumour Colonisation by Probiotic Bacterium E. coli Nissle 1917.Cancers (Basel). 2024 Aug 26;16(17):2971. doi: 10.3390/cancers16172971. Cancers (Basel). 2024. PMID: 39272829 Free PMC article. Review.
-
Biomimetic black phosphorus quantum dots-based photothermal therapy combined with anti-PD-L1 treatment inhibits recurrence and metastasis in triple-negative breast cancer.J Nanobiotechnology. 2021 Jun 13;19(1):181. doi: 10.1186/s12951-021-00932-2. J Nanobiotechnology. 2021. PMID: 34120612 Free PMC article.
-
Nafamostat mesilate, a serine protease inhibitor, suppresses interferon-gamma-induced up-regulation of programmed cell death ligand 1 in human cancer cells.Int Immunopharmacol. 2018 Jan;54:39-45. doi: 10.1016/j.intimp.2017.10.016. Epub 2017 Oct 28. Int Immunopharmacol. 2018. PMID: 29100036
-
Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy.Microb Biotechnol. 2020 May;13(3):629-636. doi: 10.1111/1751-7915.13523. Epub 2019 Dec 21. Microb Biotechnol. 2020. PMID: 31863567 Free PMC article. Review.
References
-
- Jiang N., Zhou Z., Xiong W., Chen J., Shen J., Li R., Ye R. Tumor microenvironment triggered local oxygen generation and photosensitizer release from manganese dioxide mineralized albumin-ICG nanocomplex to amplify photodynamic immunotherapy efficacy. Chin Chem Lett. 2021;32:3948–3953.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
