NLRP3 inflammasome as a therapeutic target in doxorubicin-induced cardiotoxicity: role of phytochemicals
- PMID: 40313623
- PMCID: PMC12043718
- DOI: 10.3389/fphar.2025.1567312
NLRP3 inflammasome as a therapeutic target in doxorubicin-induced cardiotoxicity: role of phytochemicals
Abstract
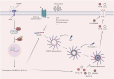
Doxorubicin (DOX) has received widespread attention as a broad-spectrum antitumor drug. However, it has been a recognized challenge that long-term DOX injections can lead to severe cardiotoxicity. There are numerous interventions to DOX-induced cardiotoxicity, and the most cost-effective is phytochemicals. It has been reported that phytochemicals have complex and diverse biological properties, facilitating the mitigation of DOX-induced cardiotoxicity. DOX-induced cardiotoxicity has numerous pathological mechanisms, and the nod-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis is one of them. This review initially presents an overview of the pathological mechanisms that underlie cardiotoxicity induced by DOX. Subsequently, we present a comprehensive elucidation of the structure and activation of the NLRP3 inflammasome. Finally, we provide a detailed summary of phytochemicals that can mitigate DOX-induced cardiotoxicity by influencing the expression of the NLRP3 inflammasome in cardiomyocytes.
Keywords: DOX-induced cardiotoxicity; NLRP3 inflammasome; myocardial injury; phytochemicals; pyroptosis.
Copyright © 2025 Zhao, Duan, Zhao, Lv, Tian, Yang and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

