Impact of mitochondrial metabolism on T-cell dysfunction in chronic lymphocytic leukemia
- PMID: 40313718
- PMCID: PMC12043688
- DOI: 10.3389/fcell.2025.1577081
Impact of mitochondrial metabolism on T-cell dysfunction in chronic lymphocytic leukemia
Abstract
T cells play a central role in anti-tumor immunity, yet their function is often compromised within the immunosuppressive tumor microenvironment, leading to cancer progression and resistance to immunotherapies. T-cell activation and differentiation require dynamic metabolic shifts, with mitochondrial metabolism playing a crucial role in sustaining their function. Research in cancer immunometabolism has revealed key mitochondrial abnormalities in tumor-infiltrating lymphocytes, including reduced mitochondrial capacity, depolarization, structural defects, and elevated reactive oxygen species. While these mitochondrial disruptions are well-characterized in solid tumors and linked to T-cell exhaustion, their impact on T-cell immunity in lymphoproliferative disorders remains underexplored. Chronic lymphocytic leukemia (CLL), the most prevalent chronic adult leukemia, is marked by profound T-cell dysfunction that limits the success of adoptive cell therapies. Emerging studies are shedding light on the role of mitochondrial disturbances in CLL-related T-cell dysfunction, but significant knowledge gaps remain. This review explores mitochondrial metabolism in T-cell exhaustion, emphasizing recent findings in CLL. We also discuss therapeutic strategies to restore T-cell mitochondrial function and identify key research gaps.
Keywords: CAR T cell; CLL (chronic lymphocytic leukemia); T-cell exhaustion; adoptive cell immunotherapy; cancer; metabolism; mitochondria.
Copyright © 2025 Gamal, Mediavilla-Varela, Kunta, Sahakian and Pinilla-Ibarz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
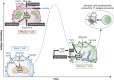
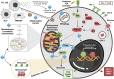
Similar articles
-
Chronic lymphocytic leukemia cells impair mitochondrial fitness in CD8+ T cells and impede CAR T-cell efficacy.Blood. 2019 Jul 4;134(1):44-58. doi: 10.1182/blood.2018885863. Epub 2019 May 10. Blood. 2019. PMID: 31076448 Free PMC article.
-
T Cells in Chronic Lymphocytic Leukemia: A Two-Edged Sword.Front Immunol. 2021 Jan 20;11:612244. doi: 10.3389/fimmu.2020.612244. eCollection 2020. Front Immunol. 2021. PMID: 33552073 Free PMC article. Review.
-
Transforming CLL management with immunotherapy: Investigating the potential of CAR T-cells and bispecific antibodies.Semin Hematol. 2024 Apr;61(2):119-130. doi: 10.1053/j.seminhematol.2024.01.001. Epub 2024 Jan 5. Semin Hematol. 2024. PMID: 38290860 Review.
-
T-cells in chronic lymphocytic leukemia: Guardians or drivers of disease?Leukemia. 2020 Aug;34(8):2012-2024. doi: 10.1038/s41375-020-0873-2. Epub 2020 May 26. Leukemia. 2020. PMID: 32457353 Free PMC article. Review.
-
T-cell dysfunction in chronic lymphocytic leukemia from an epigenetic perspective.Haematologica. 2021 May 1;106(5):1234-1243. doi: 10.3324/haematol.2020.267914. Haematologica. 2021. PMID: 33691381 Free PMC article. Review.
References
-
- Ahearne M. J., Willimott S., Piñon L., Kennedy D. B., Miall F., Dyer M. J., et al. (2013). Enhancement of CD 154/IL 4 proliferation by the T follicular helper (T fh) cytokine, IL 21 and increased numbers of circulating cells resembling T fh cells in chronic lymphocytic leukaemia. Br. J. Haematol. 162 (3), 360–370. 10.1111/bjh.12401 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

