Surface Hydration of Porous Nickel Hydroxides Facilitates the Reversible Adsorption of CO2 from Ambient Air
- PMID: 40313810
- PMCID: PMC12042040
- DOI: 10.1021/jacsau.4c01083
Surface Hydration of Porous Nickel Hydroxides Facilitates the Reversible Adsorption of CO2 from Ambient Air
Abstract
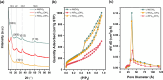
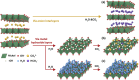
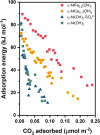
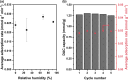
Direct air capture (DAC) under humid ambient conditions typically requires the use of organic components, with sorbents that are purely inorganic in composition for the most part operating hundreds of degrees above room temperature. In this work, we report porous metal hydroxides as a novel class of water-tolerant, oxidatively and hydrothermally stable low-temperature sorbents that exhibit competitive DAC working capacities of 1.25 mmol/g over 5 consecutive temperature swing adsorption-desorption cycles in the presence of steam and oxygen. Aqueous miscible organic solvent treatments are used to create highly porous structures with surface areas exceeding 700 m2/g that capture CO2 in the form of bicarbonates under dry conditions, and carbonates under wet conditions. Water exerts a facilitative rather than an inhibiting effect on CO2 binding, and the presence of hydrating multilayers serves to stabilize carbonate species-akin to moisture swing adsorbents-except for the fact that solvation results in a remarkable (upto 10-fold) increase, not decrease, in DAC capacity. High-valent doping with cerium is used to improve DAC capacities by amplifying surface basicity, evidencing porous nickel hydroxides specifically (and porous metal hydroxides more generally) as a novel class of robust, earth-abundant DAC sorbents.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- National Academies of Sciences, Engineering and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, 2019. - PubMed
-
- Erans M.; Sanz-Pérez E. S.; Hanak D. P.; Clulow Z.; Reiner D. M.; Mutch G. A. Direct Air Capture: Process Technology, Techno-Economic and Socio-Political Challenges. Energy Environ. Sci. 2022, 15 (4), 1360–1405. 10.1039/D1EE03523A. - DOI
-
- Damm D. L.; Fedorov A. G. Conceptual Study of Distributed CO2 Capture and the Sustainable Carbon Economy. Energy Convers. Manage. 2008, 49 (6), 1674–1683. 10.1016/j.enconman.2007.11.011. - DOI
-
- Lively R. P.; Realff M. J. On Thermodynamic Separation Efficiency: Adsorption Processes. AIChE J. 2016, 62 (10), 3699–3705. 10.1002/aic.15269. - DOI
LinkOut - more resources
Full Text Sources
