Effects of high-intensity interval training and moderate-intensity continuous training on mitochondrial dynamics in human skeletal muscle
- PMID: 40313872
- PMCID: PMC12043657
- DOI: 10.3389/fphys.2025.1554222
Effects of high-intensity interval training and moderate-intensity continuous training on mitochondrial dynamics in human skeletal muscle
Abstract
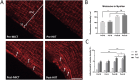
Exercise and physical activity confer health advantages, in part, by enhancing skeletal muscle mitochondrial respiratory function. The objective of this study is to analyze the impacts of high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) on the dynamics and functionality of the mitochondrial network within skeletal muscle. 20 young male participants were assigned to either HIIT or MICT group. Initial assessments of exercise-related indicators were conducted, followed by skeletal muscle biopsies from the vastus lateralis before, 1 day after, and 6 weeks post-experiment. We utilized multi-dimensional myofiber imaging to analyze mitochondrial morphology and arrangement, and assessed citrate synthase activity, complex I activity, and dynamics-related mRNA. Both training modalities increased VO2max, Wmax, citrate synthase and complex I activities, mitochondrial content, and volume density, though the changes differed between the two groups. 6 weeks training induced remodeling of the mitochondrial network within skeletal muscle. Before training, the network appeared sparse and punctate. After MICT, it adopted a grid-like structure with partially robust longitudinal connections. In contrast, HIIT resulted in a less obvious grid structure but showed a stronger longitudinally oriented network. Training also increased mRNA expression of mitochondrial fusion proteins and decreased fission protein expression, with these effects being more pronounced in HIIT. Similarly, peroxisome proliferator-activated receptor γ coactivator 1-alpha mRNA expression showed a comparable trend, though the changes differed between 1 day and 6 weeks of training. In conclusion, HIIT and MICT induce distinct mitochondrial adaptation in skeletal muscle, reflected in different network remodeling and molecular pathways. These findings may be due to HIIT's more pronounced effect on mitochondrial dynamics or respiratory function, but the study has only conducted preliminary observational experiments and further evidence is required for confirmation.
Keywords: high-intensity interval training; mitochondrial dynamics; mitochondrial network remodeling; moderate-intensity interval training; skeletal muscle.
Copyright © 2025 Li, Zhao and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


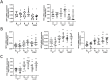
References
LinkOut - more resources
Full Text Sources

