Development of a single-dose Q fever vaccine with an injectable nanoparticle-loaded hydrogel: effect of sustained co-delivery of antigen and adjuvant
- PMID: 40314164
- PMCID: PMC12051587
- DOI: 10.1080/10717544.2025.2476144
Development of a single-dose Q fever vaccine with an injectable nanoparticle-loaded hydrogel: effect of sustained co-delivery of antigen and adjuvant
Abstract
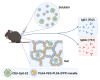
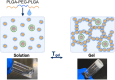
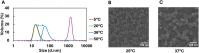
Q fever is a zoonotic infectious disease caused by Coxiella burnetii, and there is currently no FDA-approved vaccine for human use. The whole-cell inactivated vaccine Q-VAX, which is only licensed in Australia, has a risk of causing severe adverse reactions, making subunit vaccines a good alternative. However, most subunit antigens are weak immunogens and require two or more immunizations to elicit an adequate level of immunity. We hypothesized that by combining a nanoparticle to co-deliver both a protein antigen and an adjuvant, together with a hydrogel depot for sustained-release kinetics, a single-administration of a nanoparticle-loaded hydrogel vaccine could elicit a strong and durable immune response. We synthesized and characterized a protein nanoparticle (CBU-CpG-E2) that co-delivered the immunodominant protein antigen CBU1910 (CBU) from C. burnetii and the adjuvant CpG1826 (CpG). For sustained release, we examined different mixtures of PLGA-PEG-PLGA (PPP) polymers and identified a PPP solution that was injectable at room temperature, formed a hydrogel at physiological temperature, and continuously released protein for 8 weeks in vivo. Single-dose vaccine formulations were administered to mice, and IgG, IgG1, and IgG2c levels were determined over time. The vaccine combining both the CBU-CpG-E2 nanoparticles and the PPP hydrogel elicited a stronger and more durable humoral immune response than the soluble bolus nanoparticle vaccines (without hydrogel) and the free antigen and free adjuvant-loaded hydrogel vaccines (without nanoparticles), and it yielded a balanced IgG2c/IgG1 response. This study demonstrates the potential advantages of using this modular PPP hydrogel/nanoparticle system to elicit improved immune responses against infectious pathogens.
Keywords: Nanoparticle vaccine; Q fever; co-delivery; hydrogel; sustained delivery.
Conflict of interest statement
Szu-Wen Wang, D. Huw Davies, Lu Wang, and Aaron Ramirez are listed as inventors on related pending patents submitted on behalf of the University of California, Irvine. S-W Wang is an editorial board member of
Figures





References
-
- Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH, et al. . 2013. Diagnosis and management of Q fever – United States, 2013 recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 62(3):1–28. - PubMed
-
- Bian Q, Xu YH, Ma XL, Hu JY, Gu YT, Wang RX, Yuan AR, Hu WT, Huang LL, Li N, et al. . 2022. Differential dual-release bilayer microneedles loaded with aluminum adjuvants as a safe and effective vaccine platform. Adv Funct Mater. 32(29):2201952.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
