Comparative efficacy and tolerability of nutraceuticals for depressive disorder: A systematic review and network meta-analysis
- PMID: 40314175
- PMCID: PMC12094663
- DOI: 10.1017/S0033291725000996
Comparative efficacy and tolerability of nutraceuticals for depressive disorder: A systematic review and network meta-analysis
Abstract
Background: Nutraceuticals have been taken as an alternative and add-on treatment for depressive disorders. Direct comparisons between different nutraceuticals and between nutraceuticals and placebo or antidepressants are limited. Thus, it is unclear which nutraceuticals are the most efficacious.
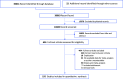
Methods: We conducted a network meta-analysis to estimate the comparative efficacy and tolerability of nutraceuticals for the treatment of depressive disorder in adults. The primary outcome was the change in depressive symptoms, as measured by the standard mean difference (SMD). Secondary outcomes included response rate, remission rate, and anxiety. Tolerability was defined as all-cause discontinuation and adverse events. Frequentist random-effect NMA was conducted.
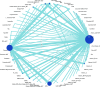
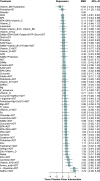
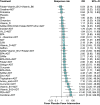
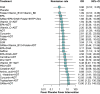
Results: Hundred and ninety-two trials involving 17,437 patients and 44 nutraceuticals were eligible for inclusion. Adjunctive nutraceuticals consistently showed better efficacy than antidepressants (ADT) alone in outcomes including SMD, remission, and response. Notable combinations were Eicosapentaenoic acid + Docosahexaenoic Acid plus ADT (EPA + DHA + ADT) (SMD 1.04, 95% confidence interval 0.64-1.44), S-Adenosyl Methionine (SAMe) + ADT (0.99, 0.31-1.68), curcumin + ADT (1.03, 0.55-1.51), Zinc + ADT (1.59, 0.63-2.55), tryptophan + ADT (1.24, 0.32-2.16), and folate + ADT (0.64, 0.17-1.10). Additionally, four nutraceutical monotherapies demonstrated superior efficacy compared to ADT: EPA + DHA (0.6, 0.32-0.88), SAMe (0.52, 0.18-0.87), curcumin (0.62, -0.17 to 1.40) and saffron (0.69, 0.34-1.04). It is noted that EPA + DHA, SAMe, and curcumin showed strong performance as either monotherapies or adjuncts to ADT. Most nutraceuticals showed comparable tolerability to placebo.
Conclusions: This extensive systematic review and NMA of nutraceuticals for treating depressive disorders indicated a number of nutraceuticals that could offer benefits, either as adjuncts or monotherapies.
Keywords: depression; network meta-analysis; nutraceuticals; randomized controlled trials.
Conflict of interest statement
The authors declare none.
Figures
References
-
- Akhondzadeh Basti, A., Moshiri, E., Noorbala, A. A., Jamshidi, A. H., Abbasi, S. H., & Akhondzadeh, S. (2007). Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: A pilot double-blind randomized trial. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 31(2), 439–442. 10.1016/j.pnpbp.2006.11.010. - DOI - PubMed
-
- Bauer, M., Pfennig, A., Severus, E., Whybrow, P. C., Angst, J., Moller, H. J., & World Federation of Societies of Biological Psychiatry. Task Force on Unipolar Depressive Disorders. (2013). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: Update 2013 on the acute and continuation treatment of unipolar depressive disorders. The World Journal of Biological Psychiatry, 14(5), 334–385. 10.3109/15622975.2013.804195. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

