Reexploring the STRESS Trial: Subgroup Postoperative Outcomes Following Methylprednisolone for Infant Heart Surgery
- PMID: 40314766
- PMCID: PMC12354065
- DOI: 10.1007/s00246-025-03875-9
Reexploring the STRESS Trial: Subgroup Postoperative Outcomes Following Methylprednisolone for Infant Heart Surgery
Abstract
Objective Assess the association between intraoperative methylprednisolone and specific postoperative outcomes among subgroups undergoing infant heart surgery.
Design: Subpopulation analyses of The Steroids to Reduce Systemic Inflammation after Infant Heart Surgery trial, a double-blind randomized placebo-controlled trial.
Setting: 24 congenital heart centers.
Patients: Infants (< 1 year old) undergoing heart surgery with cardiopulmonary bypass. Patients stratified by Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery (STAT) Mortality Category, age, gestational age, and presence of chromosomal or syndromic diagnosis (CSD).
Interventions: Methylprednisolone (30 mg/kg) versus placebo administered into cardiopulmonary bypass pump-priming fluid.
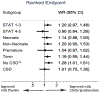
Measurements and main results: Outcomes included death, heart transplantation, mechanical circulatory support, reinterventions, and hospital length of stay. Ranked composite outcome (death, transplant, or one of 13 major complications) was compared between placebo and methylprednisolone for each subgroup using the win ratio. Methylprednisolone did not reduce odds of death, transplant, or mechanical circulatory support for any subgroup. Those receiving methylprednisolone had fewer catheterization or surgical reinterventions after STAT Category 1-3 operations [OR 0.50 (0.29-0.86)]; and fewer reoperations for bleeding among patients undergoing STAT Category 1-3 operations [OR 0.28 (0.09-0.87)], term infants [OR 0.30 (0.12-0.76)], and those without CSD [OR 0.22 (0.07-0.68)]. Length of stay was no different between methylprednisolone versus placebo. Those without chromosomal or syndromic diagnosis demonstrated a favorable association for methylprednisolone [win ratio 1.28 (1.01-1.61)] for the composite outcome.
Conclusion: Exploratory subpopulation analyses, although underpowered, suggest that methylprednisolone is not associated with significant harm and may benefit certain subpopulations.
Keywords: Cardiac surgery; Congenital heart disease; Critical care; Methylprednisolone.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Consultant for Autus Valve Technologies, Inc. (Anderson). Consultant for SpeialtyCare (J. Jacobs). For the remaining authors, no conflicts of interest are present.
Figures



References
-
- Jacobs JP, Mayer JE Jr., Pasquali SK, Hill KD, Overman DM, St Louis JD, Kumar SR, Backer CL, Tweddell JS, Dearani JA et al. : The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2019 Update on Outcomes and Quality. Ann Thorac Surg 2019, 107(3):691–704. - PubMed
-
- Graham EM, Atz AM, Butts RJ, Baker NL, Zyblewski SC, Deardorff RL, DeSantis SM, Reeves ST, Bradley SM, Spinale FG: Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery: results from a randomized trial. J Thorac Cardiovasc Surg 2011, 142(6):1523–1529. - PMC - PubMed
-
- Lomivorotov V, Kornilov I, Boboshko V, Shmyrev V, Bondarenko I, Soynov I, Voytov A, Polyanskih S, Strunin O, Bogachev-Prokophiev A et al. : Effect of Intraoperative Dexamethasone on Major Complications and Mortality Among Infants Undergoing Cardiac Surgery: The DECISION Randomized Clinical Trial. JAMA 2020, 323(24):2485–2492. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

