Current status and trends in ERCP and post-ERCP pancreatitis in Japan: a nationwide observational study
- PMID: 40314772
- PMCID: PMC12289747
- DOI: 10.1007/s00535-025-02254-8
Current status and trends in ERCP and post-ERCP pancreatitis in Japan: a nationwide observational study
Abstract
Background: Endoscopic retrograde cholangiopancreatography (ERCP) is indispensable for the management of biliary and pancreatic diseases but carries a high risk of post-ERCP pancreatitis (PEP). This study aimed to clarify the current status and temporal trends of ERCP and PEP in Japan, including preventive measures.
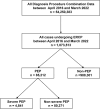
Methods: We conducted a retrospective, population-based cohort study using the Diagnosis Procedure Combination database from April 1, 2016, to March 31, 2023. Trend analyses were performed for ERCP, PEP, nonsteroidal anti-inflammatory drugs (NSAIDs), and protease inhibitors. Additionally, factors associated with PEP and severe PEP were evaluated.
Results: Among the 1,073,513 ERCP cases, PEP and severe PEP incidences were 85,212 (7.9%) and 4841 cases (0.5%), respectively. The mortality rate was 0.5% for severe PEP and 0.2% for non-severe cases. The number of ERCP procedures and the proportion of therapeutic ERCP increased over time. The incidence of PEP declined from 9.1% in the fiscal year 2016-2017 to 6.4% in the fiscal year 2022, while the incidence of severe PEP decreased from 0.5 to 0.33% over the same period. The usage rate of rectal NSAIDs increased from 16.4 to 27.6%, whereas that of protease inhibitors decreased from 70.5 to 53.5%. The administration of rectal NSAIDs at doses of 20-25 mg and 50 mg was associated with a reduced risk of severe PEP.
Conclusions: The number of ERCP procedures and the proportion of therapeutic ERCP have increased, whereas the incidences of PEP and severe PEP have decreased. Rectal NSAIDs may prevent the progression of PEP to severe disease.
Keywords: Diagnosis procedure combination; Endoscopic retrograde cholangiopancreatography; Non-steroidal anti-inflammatory drugs; Post-ERCP pancreatitis; Protease inhibitors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no conflict of interest.
Figures

References
-
- Furuta T, Kato M, Ito T, et al. 6th report of endoscopic complications: Results of the Japan Gastroenterological Endoscopy Society Survey from 2008 to 2012. Gastroenterol Endosc. 2016;58:1466–91 ((in Japanese)).
-
- Dumonceau JM, Kapral C, Aabakken L, et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127–49. - PubMed
-
- Buxbaum JL, Freeman M, Amateau SK, et al. American Society for Gastrointestinal Endoscopy guideline on post-ERCP pancreatitis prevention strategies: summary and recommendations. Gastrointest Endosc. 2023;97:153–62. - PubMed
-
- Akshintala VS, Kanthasamy K, Bhullar FA, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: an updated systematic review and meta-analysis of 145 randomized controlled trials. Gastrointest Endosc. 2023;98:1-6.e12. - PubMed
-
- Moffatt DC, Yu BN, Yie W, et al. Trends in utilization of diagnostic and therapeutic ERCP and cholecystectomy over the past 25 years: a population-based study. Gastrointest Endosc. 2014;79:615–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

