Abundant non-inclusion α-synuclein pathology in Lewy body-negative LRRK2-mutant cases
- PMID: 40314782
- PMCID: PMC12048437
- DOI: 10.1007/s00401-025-02871-w
Abundant non-inclusion α-synuclein pathology in Lewy body-negative LRRK2-mutant cases
Abstract
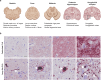
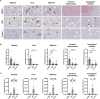
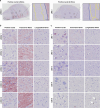
Lewy body diseases are common neurodegenerative diseases, including Parkinson's disease (PD) and dementia with Lewy bodies, which lead to both motor and non-motor symptoms. They are neuropathologically characterized by loss of neuromelanized neurons in the substantia nigra pars compacta and α-synuclein-immunopositive inclusions (Lewy bodies) in several types of neurons in the brain. A fraction of monogenic PD cases, however, represent a conundrum, as they can present with clinical Lewy body disease but do not have Lewy bodies upon neuropathological examination. For LRRK2, the presence or absence of Lewy bodies is not related to any specific mutation in the gene and different clinical presentation and neuropathology can be present even in the same family. Here, we present the first evidence of widespread α-synuclein accumulation detected with proximity ligation assay (PLA) using the MJFR14-6-4-2 antibody in six Lewy body-negative LRRK2 cases and compare the levels with five patients with neuropathologically verified Lewy body disease and six healthy controls. We show that non-inclusion aggregated α-synuclein in the form of particulate PLA signal is dominant in the LRRK2 cases, while both Lewy-like and particulate PLA signal is found in late-stage Lewy body disease. Furthermore, LRRK2 cases displayed prominent particulate PLA signal in pontocerebellar tracts and inferior olivary nuclei in the brainstem, which was not seen in idiopathic Lewy body disease cases. These results suggest that Lewy-body negative LRRK2-related PD is not associated with a lack of α-synuclein aggregation in neurons but rather a deficiency in the formation of inclusions.
Keywords: LRRK2; Lewy body disease; Neurodegeneration; Non-inclusion pathology; Proximity ligation assay; α-Synuclein.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval and informed consent: Post-mortem brain tissue was obtained from Parkinson’s UK Brain Bank (PUKBB), Imperial College London, and Oxford Brain Bank (OBB), Nuffield Department of Clinical Neurosciences in University of Oxford, in accordance with approved protocols by the Wales Research Ethics Committee (23/WA/0273) and the Ethics Committee of the University of Oxford (ref 23/SC/0241). All participants had given prior written informed consent for the brain donation. Both brain banks comply with the requirements of the Human Tissue Act 2004 and the Codes of Practice set by the Human Tissue Authority (HTA licence numbers 12275 for PUKBB and 12217 for OBB).
Figures




References
-
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ et al (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352. 10.1007/S00429-005-0025-5 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

