Development of the mechanoresponsive pericellular matrix of chondrons
- PMID: 40315311
- PMCID: PMC12047428
- DOI: 10.1126/sciadv.ado6644
Development of the mechanoresponsive pericellular matrix of chondrons
Abstract
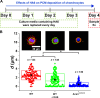
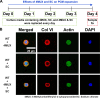
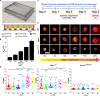
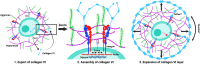
Physical properties of cartilage are conferred by the composition and ultrastructure of the extracellular matrix. This study focuses on the development of the pericellular matrix (PCM), a domain that directly contacts the chondrocyte and is a key regulator of biomechanical and biochemical signaling. Using three-dimensional cell culture, microfluidic cell compression platforms, and genetic mouse models, we demonstrated that collagen VI is initially assembled at the cell surface and then displaced to form a shell at the PCM-territorial matrix boundary. Cell surface-bound hyaluronan is crucial for the assembly process, and hyaluronan-aggrecan complexes drive displacement. Integrin adhesion is not required early but is crucial to determine the final placement of the collagen VI shell. Dynamic compression accelerated PCM maturation except in aggrecan mutants. Together, these findings provide key insights into the development of the mechanosensitive PCM and establish an in vitro platform to support studies of matrix biology in normal and disease models.
Figures

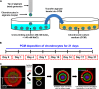
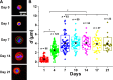

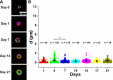
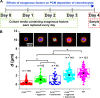
Similar articles
-
Cracking the Pericellular Matrix Code: Exploring how MMP-2, -3, and -7 influence matrix breakdown and biomechanical properties.Osteoarthritis Cartilage. 2025 Feb;33(2):241-246. doi: 10.1016/j.joca.2024.09.006. Epub 2024 Sep 23. Osteoarthritis Cartilage. 2025. PMID: 39322008
-
Aggrecan immobilizes to perineuronal nets through hyaluronan-dependent and hyaluronan-independent binding activities.J Biol Chem. 2025 Jun;301(6):108525. doi: 10.1016/j.jbc.2025.108525. Epub 2025 Apr 22. J Biol Chem. 2025. PMID: 40273987 Free PMC article.
-
Gradients in lacunar morphology and cartilage mineralization reflect the mechanical function of the mouse femoral head epiphysis.Acta Biomater. 2025 Jul 1;201:385-399. doi: 10.1016/j.actbio.2025.06.002. Epub 2025 Jun 3. Acta Biomater. 2025. PMID: 40472918
-
Autologous chondrocyte implantation in the knee: systematic review and economic evaluation.Health Technol Assess. 2017 Feb;21(6):1-294. doi: 10.3310/hta21060. Health Technol Assess. 2017. PMID: 28244303 Free PMC article.
-
From gene to mechanics: a comprehensive insight into the mechanobiology of LMNA mutations in cardiomyopathy.Cell Commun Signal. 2024 Mar 27;22(1):197. doi: 10.1186/s12964-024-01546-5. Cell Commun Signal. 2024. PMID: 38539233 Free PMC article. Review.
References
-
- Bateman J. F., Boot-Handford R. P., Lamande S. R., Genetic diseases of connective tissues: Cellular and extracellular effects of ECM mutations. Nat. Rev. Genet. 10, 173–183 (2009). - PubMed
-
- Hunter D. J., Schofield D., Callander E., The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 10, 437–441 (2014). - PubMed
-
- Sharif B., Garner R., Hennessy D., Sanmartin C., Flanagan W. M., Marshall D. A., Productivity costs of work loss associated with osteoarthritis in Canada from 2010 to 2031. Osteoarthr. Cartil. 25, 249–258 (2017). - PubMed
-
- Sengers B. G., Van Donkelaar C. C., Oomens C. W. J., Baaijens F. P. T., The local matrix distribution and the functional development of tissue engineered cartilage, a finite element study. Ann. Biomed. Eng. 32, 1718–1727 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

