A neuroimmune pathway drives bacterial infection
- PMID: 40315317
- PMCID: PMC12047438
- DOI: 10.1126/sciadv.adr2226
A neuroimmune pathway drives bacterial infection
Abstract
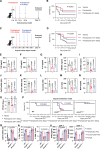
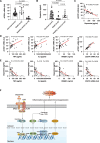
Pathogen-induced septic death presents a substantial public health challenge, with its neuroimmune mechanisms largely unexplored. Our study investigates neurotransmitter modulation of ACOD1 expression, a regulator of immunometabolism activated by bacterial lipopolysaccharide (LPS). Screening neurotransmitters identifies dopamine as a potent inhibitor of LPS-induced ACOD1 expression in innate immune cells. Mechanistically, DRD2 forms a complex with TLR4, initiating MAPK3-dependent CREB1 phosphorylation and subsequent ACOD1 transcription. Conversely, dopamine disrupts TLR4-MYD88 interaction via DRD2 without affecting the formation of the LPS-induced TLR4-MD2-CD14 complex. Enhanced ACOD1 expression induces CD274/PD-L1 production independently of itaconate, precipitating inflammation-associated immunosuppression in sepsis. Delayed administration of pramipexole, a dopamine agonist, mitigates lethality in bacterial sepsis mouse models. Conversely, the dopamine antagonist aripiprazole exacerbates sepsis mortality. Dysregulation of the dopamine-ACOD1 axis correlates with sepsis severity in patients, indicating a potential therapeutic target for modulating this neuroimmune pathway.
Figures





References
-
- Rudd K. E., Johnson S. C., Agesa K. M., Shackelford K. A., Tsoi D., Kievlan D. R., Colombara D. V., Ikuta K. S., Kissoon N., Finfer S., Fleischmann-Struzek C., Machado F. R., Reinhart K. K., Rowan K., Seymour C. W., Watson R. S., West T. E., Marinho F., Hay S. I., Lozano R., Lopez A. D., Angus D. C., Murray C. J. L., Naghavi M., Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 395, 200–211 (2020). - PMC - PubMed
-
- van der Poll T., van de Veerdonk F. L., Scicluna B. P., Netea M. G., The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 17, 407–420 (2017). - PubMed
-
- van der Poll T., Shankar-Hari M., Wiersinga W. J., The immunology of sepsis. Immunity 54, 2450–2464 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

