Targeting HMGB2 acts as dual immunomodulator by bolstering CD8+ T cell function and inhibiting tumor growth in hepatocellular carcinoma
- PMID: 40315321
- PMCID: PMC12047442
- DOI: 10.1126/sciadv.ads8597
Targeting HMGB2 acts as dual immunomodulator by bolstering CD8+ T cell function and inhibiting tumor growth in hepatocellular carcinoma
Abstract
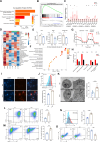
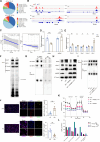
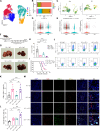
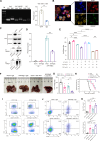
T cell exhaustion is a critical obstacle for durable treatment response in hepatocellular carcinoma (HCC). Developing drugs that control tumor growth and simultaneously bolster immune function is of great significance. Although high-mobility group box 2 (HMGB2) has been reported to be crucial to HCC prognosis, its role in the tumor microenvironment remains unclear. Here, we found HMGB2+ CD8+ T cells as being associated with immune exhaustion and resistance to anti-PD-1 treatment through single-cell RNA sequencing. Mechanistically, HMGB2 impaired the oxidative phosphorylation in CD8+ T cells and inactivated the interferon-γ response in tumor cells, reducing the antitumor effector function. Tannic acid, a specific inhibitor of HMGB2, synergized with PD-1 antibody to attenuate tumor growth and reverse T cell exhaustion. Our findings highlight the unique role of HMGB2 as an immune exhaustion associated molecule. Targeting HMGB2 on both CD8+ T cells and tumor cells contributed to promising treatment strategies for HCC.
Figures



References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). - PubMed
-
- Llovet J. M., Kelley R. K., Villanueva A., Singal A. G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R. S., Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7, 6 (2021). - PubMed
-
- Bagchi S., Yuan R., Engleman E. G., Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249 (2021). - PubMed
-
- O’Donnell J. S., Teng M. W. L., Smyth M. J., Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

