Nicotinamide Adenine Dinucleotide-Loaded Lubricated Hydrogel Microspheres with a Three-Pronged Approach Alleviate Age-Related Osteoarthritis
- PMID: 40315404
- PMCID: PMC12080321
- DOI: 10.1021/acsnano.5c01184
Nicotinamide Adenine Dinucleotide-Loaded Lubricated Hydrogel Microspheres with a Three-Pronged Approach Alleviate Age-Related Osteoarthritis
Abstract
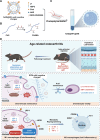
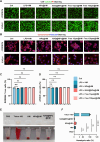
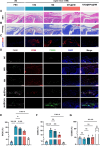
Chondrocyte senescence, synovitis, and decreased level of lubrication play pivotal roles in the pathogenesis of age-related osteoarthritis (AROA). However, there are currently no effective therapeutic interventions capable of altering the progression of OA until it reaches advanced stages, necessitating joint replacement. In this study, lubricious and drug-loaded hydrogel microspheres were designed and fabricated by utilizing microfluidic technology for radical polymerization of chondroitin sulfate methacrylate and incorporating nicotinamide adenine dinucleotide (NAD)-loaded liposomes modified with lactoferrin that are positively charged. Mechanical, tribological, and drug release analyses demonstrated enhanced lubrication properties and an extended drug dissemination time for the NAD@NPs@HM microspheres. In vitro assays unveiled the ability of NAD@NPs@HM to counteract chondrocyte senescence. RNA sequencing analysis, untargeted metabolomics analysis, and in vitro experiments on macrophages revealed that NAD@NPs@HM can regulate the metabolic reprogramming of synovial macrophages, promoting their repolarization from the M1 to M2 phenotype, thereby alleviating synovitis. Intra-articular injection of NAD@NPs@HM in aged mice reduced the mechanisms associated with AROA. These results suggest that NAD@NPs@HM may provide extended drug release, improved joint lubrication leading to better gait, and attenuation of AROA pathogenic processes, indicating its potential as a therapeutic approach for AROA.
Keywords: age-related osteoarthritis; cellular senescence; lubrication; macrophage reprogramming; nicotinamide adenine dinucleotide.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

