Protoporphyrin IX-Derived Ruthenium(II) Complexes for Photodynamic Therapy in Gastric Cancer Cells
- PMID: 40315445
- PMCID: PMC12093383
- DOI: 10.1021/acs.inorgchem.5c00896
Protoporphyrin IX-Derived Ruthenium(II) Complexes for Photodynamic Therapy in Gastric Cancer Cells
Abstract
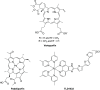
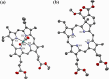
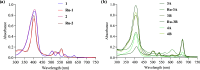
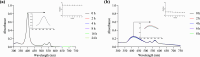
In recent years, photodynamic therapy (PDT) has emerged as a promising alternative to classical chemotherapy for treating cancer. PDT is based on a nontoxic prodrug called photosensitizer (PS) activated by light at the desired location. Upon irradiation, the PS reacts with the oxygen present in the tumor, producing cytotoxic reactive oxygen species (ROS). Compounds with highly conjugated π-bond systems, such as porphyrins and chlorins, have proven to be excellent light scavengers, and introducing a metal atom in their structure improved the generation of ROS. In this work, a series of tetrapyrrole-ruthenium(II) complexes derived from protoporphyrin IX and the commercial drug verteporfin were designed as photosensitizers for PDT. The complexes were almost nontoxic on human gastric cancer cells under dark conditions, revealing remarkable cytotoxicity upon irradiation with light. The ruthenium atom in the central cavity of the chlorin ligand allowed combined mechanisms in photodynamic therapy, as both singlet oxygen and superoxide radicals were detected. Additionally, one complex produced large amounts of singlet oxygen under hypoxic conditions. Biological assays demonstrated that the ruthenium derivatives caused cell death through a caspase 3 mediated apoptotic pathway and via CHOP, an endoplasmic reticulum stress-inducible transcription factor involved in apoptosis and growth arrest.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


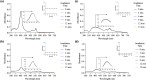


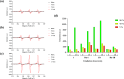
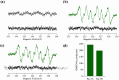



References
-
- Oluwajembola A. M.; Cleanclay W. D.; Onyia A. F.; Chikere B. N.; Zakari S.; Ndifreke E.; De Campos O. C. Photosensitizers in photodynamic therapy: An advancement in cancer treatment. Results Chem. 2024, 10, 101715 10.1016/j.rechem.2024.101715. - DOI
-
- Alderden R. A.; Hall M. D.; Hambley T. W. The Discovery and Development of Cisplatin. J. Chem. Educ. 2006, 83 (5), 728 10.1021/ed083p728. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

