Dissecting the immune landscape in pediatric high-grade glioma reveals cell state changes under therapeutic pressure
- PMID: 40315846
- PMCID: PMC12147851
- DOI: 10.1016/j.xcrm.2025.102095
Dissecting the immune landscape in pediatric high-grade glioma reveals cell state changes under therapeutic pressure
Abstract
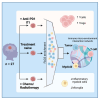
Pediatric high-grade gliomas (pHGGs) are among the most lethal childhood tumors. While therapeutic approaches were largely adapted from adult treatment regime, significant biological differences between pediatric and adult gliomas exist, which influence the immune microenvironment and may contribute to the limited response to current pHGG treatment strategies. We provide a comprehensive transcriptomic analysis of the pHGG immune landscape using single-cell RNA sequencing and spatial transcriptomics. We analyze matched malignant, myeloid, and T cells from patients with pediatric diffuse high-grade glioma (HGG) or high-grade ependymoma, examining immune microenvironment distinctions after chemo-/radiotherapy, immune checkpoint inhibition treatment, and by age. Our analysis reveals differences in the proportions of pediatric myeloid subpopulations compared to adult counterparts. Additionally, we observe significant shifts toward immune-suppressive environments following cancer therapy. Our findings offer valuable insights into potential immunotherapy targets and serve as a robust resource for understanding immune microenvironmental variations across HGG age groups and treatment regimens.
Keywords: immuno-oncology; pediatric high-grade glioma; single-cell RNA-seq; tumor microenvironment; tumor-infiltrating immune cell biology.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.G.F. is a consultant for Twentyeight-Seven Therapeutics and Blueprint Medicines. K.W.W. is a co-founder member of Immunitas Therapeutics. K.W.W. serves on the scientific advisory board of TCR2 Therapeutics, T-Scan Therapeutics, SQZ Biotech, DEM BioPharma, Bisou Bioscience Company, and Nextech Invest and received sponsored research funding from Novartis.
Figures




References
-
- Liu I., Jiang L., Samuelsson E.R., Marco Salas S., Beck A., Hack O.A., Jeong D., Shaw M.L., Englinger B., LaBelle J., et al. The landscape of tumor cell states and spatial organization in H3-K27M mutant diffuse midline glioma across age and location. Nat. Genet. 2022;54:1881–1894. doi: 10.1038/s41588-022-01236-3. - DOI - PMC - PubMed
-
- Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. - DOI - PMC - PubMed
-
- Mathewson N.D., Ashenberg O., Tirosh I., Gritsch S., Perez E.M., Marx S., Jerby-Arnon L., Chanoch-Myers R., Hara T., Richman A.R., et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell. 2021;184:1281–1298.e26. doi: 10.1016/j.cell.2021.01.022. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

