Metaplasticity and continual learning: mechanisms subserving brain computer interface proficiency
- PMID: 40315903
- PMCID: PMC12101542
- DOI: 10.1088/1741-2552/add37b
Metaplasticity and continual learning: mechanisms subserving brain computer interface proficiency
Abstract
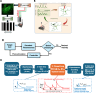
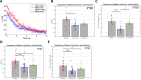
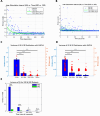
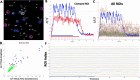
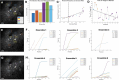
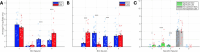
Objective.Brain computer interfaces (BCIs) require substantial cognitive flexibility to optimize control performance. Remarkably, learning this control is rapid, suggesting it might be mediated by neuroplasticity mechanisms operating on very short time scales. Here, we propose a meta plasticity model of BCI learning and skill consolidation at the single cell and population levels comprised of three elements: (a) behavioral time scale synaptic plasticity (BTSP), (b) intrinsic plasticity (IP) and (c) synaptic scaling (SS) operating at time scales from seconds to minutes to hours and days. Notably, the model is able to explainrepresentational drift-a frequent and widespread phenomenon that adversely affects BCI control and continued use.Approach.We developed an all-optical approach to characterize IP, BTSP and SS with single cell resolution in awake mice using fluorescent two photon (2P) GCaMP7s imaging and optogenetic stimulation of the soma targeted ChRmineKv2.1. We further trained mice on a one-dimensional BCI control task and systematically characterized within session (seconds to minutes) learning as well as across sessions (days and weeks) with different neural ensembles.Main results.On the time scale of seconds, substantial BTSP could be induced and was followed by significant IP over minutes. Over the time scale of days and weeks, these changes could predict BCI control proficiency, suggesting that BTSP and IP might be complemented by SS to stabilize and consolidate BCI control.Significance.Our results provide early experimental support for a meta plasticity model of continual BCI learning and skill consolidation. The model predictions may be used to design and calibrate neural decoders with complete autonomy while considering the temporal and spatial scales of plasticity mechanisms. With the power of modern-day machine learning and artificial Intelligence, fully autonomous neural decoding and adaptation in BCIs might be achieved with minimal to no human intervention.
Keywords: BCI; calcium imaging; cognitive control; learning; optogenetics; synaptic plasticity.
Creative Commons Attribution license.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
