Cost-effectiveness of Lynch Syndrome Screening in Colorectal Cancer: Universal Germline vs Sequential Screening
- PMID: 40315972
- PMCID: PMC12354148
- DOI: 10.1016/j.cgh.2025.03.006
Cost-effectiveness of Lynch Syndrome Screening in Colorectal Cancer: Universal Germline vs Sequential Screening
Abstract
Background & aims: Testing all colorectal cancers (CRCs) for mismatch repair status to evaluate for Lynch syndrome (LS) has been recommended for years. Owing to attrition in the multistep diagnostic testing pathway, most qualifying patients still do not receive genetic testing for LS. This leads to missed diagnoses and preventable cancer incidence. To tackle this, we previously reported a systems approach that resulted in a dramatic increase in the identification of patients with LS. We aim to evaluate the cost-effectiveness of this intervention compared with both real-world pre-intervention experience and with upfront germline testing of all CRC probands.
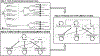
Methods: We employed data from the Prospective Lynch Syndrome Database, the National Cancer Institute Surveillance, Epidemiology, and End Results program, and pre-/post-intervention cohort studies to build lifetime Markov cohorts of CRC probands, testing 3 strategies: (1) current standard-of-care; (2) optimized standard-of-care; and (3) upfront germline testing. The primary outcome was the incremental cost-effectiveness ratio (ICER) in $ per quality-adjusted life-year (QALY) from the United States health system perspective.
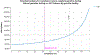
Results: Strategies #1 to #3 accrued 11.97, 11.98, and 11.99 discounted QALYs at discounted costs of $100,610, $100,980, and $102,290, respectively. The pairwise ICERs on the frontier were $34,500/QALY (95% credible interval [CI], $28,400-$44,200) and $98,500/QALY (95% CI, $73,700-$216,000), respectively. The cost-effectiveness of #3 vs #1 was $70,300/QALY (95% CI, $54,600-$92,500). Current standard-of-care was favored in 0.0% of 10,000 Monte Carlo iterations.
Conclusions: Current clinical practice is cost-ineffective. Prospective intervention to dramatically increase LS testing (ie, to reach a threshold of >75%) or, if this level cannot be reached, upfront germline testing are cost-effective interventions that improve quality-adjusted life expectancy.
Keywords: Colorectal Cancer; Cost-effectiveness; Diagnosis; Lynch Syndrome.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement: In the past three years, HMK has received options for Element Science and Identifeye and payments from F-Prime for advisory roles. He is a co-founder of and holds equity in Hugo Health, Refactor Health, and ENSIGHT-AI. He is associated with research contracts through Yale University from Janssen, Kenvue, Novartis, and Pfizer.
Figures
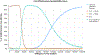
References
-
- Network NCC. NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric. Version 3. 2024. National Comprehensive Cancer Network, Inc. 10/31/2024. - PubMed
-
- Burn J, Sheth H, Elliott F, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet (London, England) 2020;395(10240):1855–1863. (In eng). DOI: 10.1016/s0140-6736(20)30366-4. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

