Improved Medication communication and Patient involvement At Care Transitions (IMPACT-care): study protocol for a pre-post intervention trial in older hospitalised patients
- PMID: 40316362
- PMCID: PMC12049937
- DOI: 10.1136/bmjopen-2025-099547
Improved Medication communication and Patient involvement At Care Transitions (IMPACT-care): study protocol for a pre-post intervention trial in older hospitalised patients
Abstract
Introduction: Care transitions, particularly hospital discharge, present significant risks to patient safety. Deficient medication-related discharge communication is a major contributor, posing substantial risk of harm to older patients. This protocol outlines the Improved Medication communication and Patient involvement At Care Transitions (IMPACT-care) intervention study, designed to evaluate the effects of a multifaceted intervention for older hospitalised patients on medication-related discharge communication compared with usual hospital care.
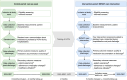
Methods and analysis: A pre-post intervention study will be conducted in two surgical and one geriatric ward of a university hospital in Sweden. The study will begin with a control period delivering usual care, followed by a training period and then an intervention period. The intervention comprises four components performed by clinical pharmacists: (1) information package provided to patients and/or informal caregivers, (2) preparation of medication-related discharge documentation, (3) facilitation of discharge communication and (4) follow-up call to patients or their informal caregiver. Eligible participants are aged ≥65 years, manage their own medications independently or with informal caregiver support, and are admitted to the study wards. Each study period (control and intervention) will last until 115 patients have been included. The primary outcome is the quality of medication-related discharge documentation, assessed using the Complete Medication Documentation at Discharge Measure (CMDD-M). Secondary outcomes include patients' perceptions of knowledge and involvement in discharge medication communication, and their sense of security in managing medication post-discharge; adherence to medication changes from hospitalisation that persist after discharge; and unplanned healthcare visits following discharge. A process evaluation is planned to explore how the intervention was implemented. Patient inclusion began in September 2024.
Ethics and dissemination: The study protocol has been approved by the Swedish Ethical Review Authority (registration no.: 2023-03518-01 and 2024-04079-02). Results will be published in open-access international peer-reviewed journals, and presented at national and international conferences.
Trial registration number: NCT06610214.
Keywords: Clinical Protocols; Health Services for the Aged; Hospital to Home Transition; Medication Adherence; Patient-Centered Care; Pharmacists.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- United Nations Department of Economic and Social Affairs, Population Division . World population prospects 2022: summary of results. New York, NY: 2022. https://www.un.org/development/desa/pd/content/World-Population-Prospect... Available.
-
- U.S. Centers for Disease Control and Prevention . Persons with hospital stays in the past year, by selected characteristics: United States, selected years. Atlanta: https://www.cdc.gov/nchs/data/hus/2019/040-508.pdf Available.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
