The efficacy and safety of anti-amyloid monoclonal antibody versus acetylcholinesterase inhibitor with an in-depth analysis across genotypes and disease stages: a systematic review and meta-analysis
- PMID: 40316479
- PMCID: PMC12413725
- DOI: 10.1016/j.tjpad.2025.100195
The efficacy and safety of anti-amyloid monoclonal antibody versus acetylcholinesterase inhibitor with an in-depth analysis across genotypes and disease stages: a systematic review and meta-analysis
Abstract
Background: To date, studies have not compared the efficacy and safety of monoclonal antibodies (mABs) with acetylcholinesterase inhibitors (AChEIs).
Methods: Five electronic databases were systemic searched from inception to 10 November 2024 for double-blinded randomized controlled trial (RCT) of patients diagnosed with MCI or mild AD treated with mABs or AChEIs for at least 6 months. The primary outcome was change in cognitive function, measured by the Alzheimer's Disease Assessment Scale-cognitive subscale 14-item (ADAS-Cog) and Clinical Dementia Rating Scale-Sum of Boxes (CDR-SOB). The secondary outcomes were acceptability, tolerability, serious adverse events (SAE), and all -cause mortality. For mABs, amyloid-related imaging abnormalities-edema (ARIA-E), and amyloid-related imaging abnormalities-hemorrhage (ARIA-H) were also assessed. Subgroup analyses included (i) MCI versus mild AD; (ii) with versus without concomitant AD medications; and (iii) Apolipoprotein E (ApoE4) carriers versus non-carriers. Data were pooled using a random effects model within a Bayesian framework.
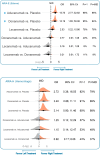
Results: There were 8010 participants (mean age: 71.5 years) across seven mAB trials, and 4993 participants (mean age:70.7 years) in nine AChEI trials. When compared to placebo, only mABs, not AChEIs, were associated with a slower progression of cognitive decline on CDR-SOB (mean difference -0.41 (95 % credible interval -0.61 to -0.22); minimally important difference (MID) -1) and ADAS-Cog (-1.35 (-2.36 to -0.36), MID -2); however, these benefits of mABs did not reach MID across the two cognitive measurements. Besides, mABs were associated with a slower progression of cognitive decline on CDR-SOB (-0.30 (-0.60 to -0.001)) than AChEIs, although mABs and AChEIs did not differ across safety outcomes, including acceptability, tolerability, SAE, and all-cause mortality. Further analysis of mABs indicated that their efficacy did not differ by disease stage, concomitant AD medications, or APOE4 carrier status. However, APOE4 homozygotes carriers were associated with a 5.53-fold (2.48 to 13.07) increased odds of developing ARIA-E compared to non-carriers. Finally, lecanemab demonstrated relatively better efficacy and a more favorable profile on ARIA-E compared to aducanumab and donanemab.
Conclusions: mABs were associated with a slower progression of cognitive decline than AChEIs; however, this effect did not reach the MID. The incidence of ARIA-E with mABs was associated with APOE4 carrier status and was not indicative of treatment efficacy.
Keywords: Alzheimer's disease; Apolipoprotein E4; Cholinesterase inhibitors; Cognitive function; Monoclonal antibodies.
Copyright © 2025 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

