CD56 expression modulates NAD+ metabolic landscape and predicts sensitivity to anti-CD38 therapies in multiple myeloma
- PMID: 40316562
- PMCID: PMC12048669
- DOI: 10.1038/s41408-025-01284-y
CD56 expression modulates NAD+ metabolic landscape and predicts sensitivity to anti-CD38 therapies in multiple myeloma
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
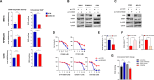
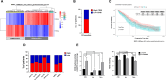
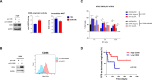
References
-
- Cottini F, Rodriguez J, Hughes T, Sharma N, Guo L, Lozanski G, et al. Redefining CD56 as a biomarker and therapeutic target in multiple myeloma. Mol Cancer Res. 2022;20:1083–95. - PubMed
-
- Flores-Montero J, de Tute R, Paiva B, Perez JJ, Böttcher S, Wind H, et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytom B Clin Cytom. 2016;90:61–72. - PubMed
-
- Giorgetti G, Becherini P, Maroto-Martin E, Fenoglio D, Soncini D, Martinuzzi C, et al. Higher CD56 expression on multiple myeloma cells increases CD38 expression, reduces intracellular NAD+ levels, and enhances the efficacy of daratumumab-based treatment strategies. Blood. 2023;142:1951
-
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–86. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

