Ministring DNA (msDNA): a novel linear covalently-closed DNA with enhanced stability for gene and cell therapy applications
- PMID: 40316641
- PMCID: PMC12048660
- DOI: 10.1038/s41598-025-98730-5
Ministring DNA (msDNA): a novel linear covalently-closed DNA with enhanced stability for gene and cell therapy applications
Abstract
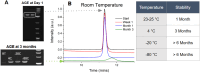
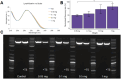
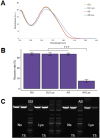
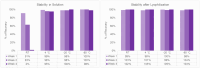
The quality and fidelity of DNA vectors used in genetic medicine and gene therapy either as starting material for manufacturing or as therapeutic ingredients play a critical role in determining ultimate clinical success. Ministring DNA (msDNA), is a novel minivector that is a linear covalently-closed (LCC) double-stranded DNA molecule devoid of immunogenic bacterial sequences (e.g., origin of replication, antibiotic resistant cassette). Similar to traditional plasmids, msDNA is manufactured in vivo in E. coli and therefore benefits from the scalability of E. coli -based systems and the ~ 1000-fold enhanced fidelity conferred by the mismatch repair (MMR) mechanism. In this paper, we address the improved stability of msDNA. We show that due to the torsion-free structure, msDNA is more stable to chemical and mechanical stress than conventional plasmid DNA. Moreover, we demonstrate that lyophilization can further improve the long-term stability of msDNA, reducing the need for cold chain storage. Therefore, we propose that msDNA can be a new paradigm for genetic medicine by offering genetic material with lower immunogenicity, reduced risk of insertional mutagenesis, and higher fidelity and stability.
Keywords: Chemical and mechanical stability; Gene therapy; Keywords; Linear covalently closed DNA; Lyophilization; Ministring DNA.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors are employees and own stock in Mediphage Bioceuticals, Inc. Ethical approval: No ethical approval was required as no human or animal experiments we carried out.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

