Autoimmune encephalitis-associated epilepsy
- PMID: 40316743
- PMCID: PMC12188692
- DOI: 10.1038/s41582-025-01089-4
Autoimmune encephalitis-associated epilepsy
Abstract
Autoimmune encephalitis (AE), defined by clinical criteria and its frequent association with neural autoantibodies, often manifests with seizures, which usually stop with immunotherapy. However, a subset of encephalitic conditions present with recurrent seizures that are resistant to immunotherapy. Three primary neurological constellations that fall within this subset are discussed in this Perspective: temporal lobe epilepsy with antibodies against glutamic acid decarboxylase, epilepsy in the context of high-risk paraneoplastic antibodies, and epilepsy following adequately treated surface antibody-mediated AE. These entities all share a common mechanism of structural injury and potentially epileptogenic focal neural loss, often induced by cytotoxic T cells. Recently, we have proposed conceptualizing these conditions under the term autoimmune encephalitis-associated epilepsy (AEAE). Here, we discuss the new concept of AEAE as an emerging field of study. We consider the clinical characteristics of patients who should be investigated for AEAE and highlight the need for judicious use of traditional epilepsy therapeutics alongside immunotherapeutic considerations that are of uncertain and incomplete efficacy for this group of disorders. Last, we discuss future efforts needed to diagnose individuals before structural epileptogenesis has superseded inflammation and to develop improved therapeutics that target the specific immunological or functional disturbances in this entity.
© 2025. Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
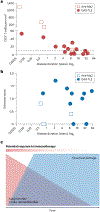

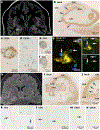


References
-
- Fisher RS et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46, 470–472 (2005). - PubMed
-
- Eeg-Olofsson O, Prchal JF & Andermann F Abnormalities of T-lymphocyte subsets in epileptic patients. Acta Neurol. Scand 72, 140–144 (1985). - PubMed
-
- Aarli JA & Fontana A Immunological aspects of epilepsy. Epilepsia 21, 451–457 (1980). - PubMed
-
- Karpiak SE Jr., Bowen FP & Rapport MM Epileptiform activity induced by antiserum to synaptic membrane. Brain Res. 59, 303–310 (1973). - PubMed
-
- Mihailović LT & Cupić D Epileptiform activity evoked by intracerebral injection of anti-brain antibodies. Brain Res. 32, 97–124 (1971). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

