Developing a Standardised Dataset for Natural History Studies in Fibrous Dysplasia/McCune-Albright Syndrome
- PMID: 40316789
- PMCID: PMC12048454
- DOI: 10.1007/s00223-025-01379-5
Developing a Standardised Dataset for Natural History Studies in Fibrous Dysplasia/McCune-Albright Syndrome
Abstract
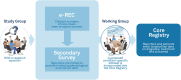
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare and complex condition caused by somatic variants in the GNAS gene that lead to a wide clinical spectrum. The diagnostic process and therapeutic pathway vary per centre and therefore international harmonisation of data collection should be pursued. To understand the diagnostic pathways and clinical outcomes of patients with FD/MAS reported on an electronic-reporting tool (e-REC) across European centres to guide the develop a condition-specific module within the European Registries for Rare Endocrine and Bone conditions. Centres that reported new cases on e-REC between October 2019 and May 2021 were approached to complete a survey in May 2021. Fifty-eight cases were included. Median age at presentation was 20 years (range, 0, 72). Of the 58 included cases, the presentation type was isolated craniofacial FD in 19 (33%), monostotic FD in 15 (26%), polyostotic FD in 10 (17%), and MAS in 13 (13%). Standardised questionnaires to assess pain and quality of life were used routinely in 21/58 patients (36%). The majority of patients had more than one healthcare provider, with great diversity in the specialty of the coordinating physician. A standardised dataset module for FD/MAS was developed through collaboration with the FD/MAS study group, incorporating expert consensus and clinical insights. Key variables were identified to capture essential diagnostic, clinical, and patient-reported outcomes. The diagnostic path for patients with FD/MAS across European expert centres is variable. The outcomes of this study allowed the building of the first international FD/MAS-specific data collection.
Keywords: Data collection; Fibrous dysplasia; McCune-Albright syndrome; Rare bone disease; Registries.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: ALPZ declares that Leiden University Medical Center received funding from the European Union’s Health Programme to host Project ‘946831 / EuRR-Bone’ and project ‘777215 / EuRRECa’, OOBD declares that Leiden University Medical Center received funding from the European Union’s Health Programme to host Project ‘946831 / EuRR-Bone’ and project ‘777215 / EuRRECa’, JB declares that University of Glasgow received funding from the European Union’s Health Programme to host project ‘777215 / EuRRECa’ and participate in project ‘946831 / EuRR-Bone’, NA declares no competing interests, MCC declares no competing interests, MC declares that Leiden University Medical Center received funding from the European Union’s Health Programme to host Project ‘946831 / EuRR-Bone’ and project ‘777215 / EuRRECa’, GD declares no competing interests, CG declares that Ruhr-University Bochum received funding from the European Union’s Health Programme to participate in Project ‘946831 / EuRR-Bone’, MKJ declares that University of Oxford received funding from the European Union’s Health Programme to participate in Project ‘946831 / EuRR-Bone’, HM declares that University of Glasgow received funding from the European Union’s Health Programme to host project ‘777215 / EuRRECa’ and participate in project ‘946831 / EuRR-Bone’, SWM declares that Leiden University Medical Center received funding from the European Union’s Health Programme to host Project ‘946831 / EuRR-Bone’ and project ‘777215 / EuRRECa’, DOC declares no competing interests, LDS declares no competing interests, LS declares no competing interests, AVS declares no competing interests, DT declares no competing interests, PBW declares that Leiden University Medical Center received funding from the European Union’s Health Programme to host Project ‘946831 / EuRR-Bone’ and project ‘777215 / EuRRECa’, RC declares no competing interests, SFA declares that Leiden University Medical Center and University of Glasgow received funding from the European Union’s Health Programme to host Project ‘946831 / EuRR-Bone’ and project ‘777215 / EuRRECa’, NMAD declares that Leiden University Medical Center received funding from the European Union’s Health Programme to host Project ‘946831 / EuRR-Bone’ and project ‘77721 EuRRECa’. Human and Animal Rights and Informed Consent: The project was approved by the Information Governance authorities at the NHS Greater Glasgow & Clyde Health Board and the National Research Ethics Service in the UK (e-REC REC Reference 17/WS/0178; Core Registry REC Reference 18//WS/0205) and later at the Leiden University Medical Center (e-REC project ID 133036; Core Registry protocol nWMODIV2_2024018, project ID 130850).
Figures

References
-
- Leet AI, Chebli C, Kushner H, Chen CC, Kelly MH, Brillante BA, Robey PG, Bianco P, Wientroub S, Collins MT (2004) Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res 19(4):571–577 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

