Cost-Effectiveness Analysis of Nirsevimab for the Prevention of Respiratory Syncytial Virus among Italian Infants
- PMID: 40317387
- PMCID: PMC12144043
- DOI: 10.1007/s40261-025-01437-8
Cost-Effectiveness Analysis of Nirsevimab for the Prevention of Respiratory Syncytial Virus among Italian Infants
Abstract
Background and objective: Respiratory syncytial virus (RSV) is a major global cause of childhood respiratory infections, globally linked to significant morbidity and mortality, particularly leading in hospitalizations and death among infants below 1 year of age. A cost-effectiveness analysis was conducted to estimate the economically justifiable price (EJP) of nirsevimab, a new prophylaxis strategy protecting all infants against RSV lower respiratory tract infections (LRTIs), compared with a strategy consisting of palivizumab, protecting only high-risk infants and no preventive intervention for others.
Methods: A static decision tree model previously published to evaluate the clinical and economic burden of RSV in Italy was used to determine the EJP of nirsevimab for the prevention of RSV medically attended lower respiratory tract infections (RSV-MA-LRTIs) in all infants experiencing their first RSV season, to become a cost-effective alternative compared with palivizumab only in high-risk infants and no preventive intervention for others. The EJP was estimated considering three different willingness-to-pay (WTP) thresholds. The National Health Service (NHS) perspective was considered in the base-case. Direct costs considered in the analysis were acquisition and administration costs of prophylaxis, costs of managing RSV infection (inpatient and outpatient care, and emergency department visits) and costs of handling complications following hospitalization per RSV event. Indirect costs were evaluated in the scenario analysis as productivity loss due to premature death for RSV infection. A discount rate of 3.0% was applied only to mid-long-term costs and outcomes.
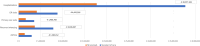
Results: From the NHS perspective, over the first RSV season, nirsevimab in an all-infants population could be a cost-effective approach compared with palivizumab only in high-risk infants, with an EJP equal to €267, €365, and €400 considering a WTP threshold of €0, €22,000, and €30,000 per QALY saved, respectively. Considering only the palivizumab-eligible population, the model estimated that nirsevimab could be a cost-effective approach with an EJP equal to €3,467, €3,633, and €3,694 considering a WTP threshold of €0, €22,000, and €30,000 per QALY saved, respectively.
Conclusions: A prophylaxis strategy against RSV infection targeting all infants with nirsevimab could represent a cost-effective option for both NHS and societal perspectives, and supports the implementation and the equity of RSV prevention for all infants.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement. This study was funded by Sanofi and AstraZeneca, and Sanofi authors (Muzii B. and Soudani S.) were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. Conflicts of interest: Muzii B and Soudani S are employees of Sanofi and may have shares and/or stock options of the Company. Bini C., Cazzato D., Bozzola E., Midulla F., Baraldi E., Bonanni P., Boccalini S., and Orfeo L. declare that they have no conflicts of interest. Marcellusi A. is an Editorial Board member of Clinical Drug Investigation. Dr. Andrea Marcellusi was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Ethics approval: Not applicable Consent to participate: Not applicable Consent for publication: Not applicable Code availability: Not applicable Availability of data and material: Not applicable Author contributions: Concept and design: Bini, Marcellusi, Muzii, and Soudani. Acquisition of data: Bini, Marcellusi, Muzii, Soudani, Bozzola, Midulla, Baraldi, Bonanni, Boccalini, and Orfeo. Analysis and interpretation of data: Bini, Marcellusi, Cazzato, Muzii, Soudani, Bozzola, Midulla, Baraldi, Bonanni, Boccalini, and Orfeo. Drafting of the manuscript: Bini, Cazzato, Muzii, and Soudani. Critical revision of the paper for important intellectual content: Bini, Marcellusi, Cazzato, Muzii, Soudani, Bozzola, Midulla, Baraldi, Bonanni, Boccalini, and Orfeo. Administrative, technical, or logistic support: Bini, Marcellusi, and Muzii. Supervision: Bini, Marcellusi, Muzii, and Soudani. All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Figures

References
-
- Bini C, Marcellusi A, Muzii B, Soudani S, Kieffer A, Beuvelet M, et al. Economic and clinical burden associated with respiratory syncytial virus (RSV) and expected impact of universal immunization with nirsevimab among all infants in their first RSV season against standard of care in Italy 2023. https://www.ispor.org/docs/default-source/euro2023/isporeurope23biniee69.... Accessed 6 Mar 2025.
-
- Circolare del Ministero della Salute. OGGETTO: Misure di prevenzione e immunizzazione contro il virus respiratorio sinciziale (VRS). https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2024&co.... Accessed 6 Mar 2025.
-
- GU n. 124 del 30 maggio 2015. https://www.gazzettaufficiale.it/eli/gu/2015/05/30/124/sg/pdf. Accessed 6 Mar 2025.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

