Performance Evaluation of Five Real-Time PCR Assays for the Detection of Candida auris DNA
- PMID: 40317536
- PMCID: PMC12048891
- DOI: 10.1111/myc.70065
Performance Evaluation of Five Real-Time PCR Assays for the Detection of Candida auris DNA
Abstract
Objectives: This study aimed to systematically evaluate and compare the performance of two laboratory-developed assays (LDAs) and three commercially available real-time PCR assays for the detection of Candida auris. The analytical sensitivity, specificity and limit of detection (LOD) of each assay were assessed, alongside their clinical sensitivity in identifying C. auris colonisation.
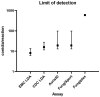
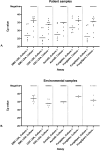
Methods: Ten C. auris strains representing five clades, as well as genetically related yeasts, common yeast species, and dermatophytes, were used to assess assay sensitivity and cross reactivity. Clinical and environmental samples were collected from patients during an outbreak and tested with three commercial PCR assays (AurisID, Fungiplex, FungiXpert) and two LDAs (CDC LDA, EMC LDA). LOD was determined using Probit analysis. Diagnostic sensitivity was evaluated by comparing the detection rate of each individual assay to the total detection rate of all assays combined.
Results: The EMC LDA exhibited the highest analytical sensitivity, with a LOD of 8 conidia/reaction, followed by CDC LDA (16 conidia/reaction), AurisID and FungiXpert (19 conidia/reaction), and Fungiplex (596 conidia/reaction). Specificity testing revealed cross-reactivity in the CDC LDA and AurisID assays with C. pseudohaemulonii at high conidia levels, while no cross-reactivity was observed in the other assays. EMC LDA showed the highest clinical sensitivity (100%), whereas Fungiplex had the lowest positivity rate (71%). No false positives were observed in negative control swabs for any assay.
Conclusions: Real-time PCR is a crucial tool for the rapid and sensitive detection of C. auris , especially in clinical settings where timely identification is essential for effective patient management and infection control. Numerous PCR assays are available for this purpose; however, our study demonstrates that the sensitivity of these assays can vary significantly. The observed differences underscore the importance of establishing international reference standards and proficiency panels to enhance the accuracy and comparability of assay performance across different studies and laboratories.
Keywords: Candida; Candida spp; PCR.
© 2025 The Author(s). Mycoses published by Wiley‐VCH GmbH.
Conflict of interest statement
J.B.B. reports grants from Gilead Sciences, BioMérieux, lectures for Gilead Sciences and Pfizer, and advisory board for Gilead Sciences; all grants are paid to the institute. E.F.J.M. discloses speaker engagements for Gilead Sciences, advisory board fees from Pfizer, and research funding from MundiPharma and Scynexis. S.J.M. reports grants from Gilead Sciences and Mundipharma. P.E.V. reports consulting fees, advisory board and speaker fees from Gilead Sciences and consulting fees from Mundipharma, all paid to the institute. All authors declare no conflicts of interest.
Figures

References
-
- Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., and Yamaguchi H., “ Candida auris sp. Nov., a Novel Ascomycetous Yeast Isolated From the External Ear Canal of an Inpatient in a Japanese Hospital,” Microbiology and Immunology 53, no. 1 (2009): 41–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

