Tubulin Polymerization Promoting Proteins: Functional Diversity With Implications in Neurological Disorders
- PMID: 40317801
- PMCID: PMC12047068
- DOI: 10.1002/jnr.70044
Tubulin Polymerization Promoting Proteins: Functional Diversity With Implications in Neurological Disorders
Abstract
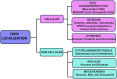
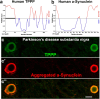
Tubulin Polymerization Promoting Proteins (TPPPs) are highly conserved across species but remain poorly understood. There are three TPPP genes in humans, with only one homologous TPPP gene in invertebrates, such as Drosophila and C. elegans. The human TPPP (TPPP1/p25/p25α) is enriched in the brain and shares sequence similarities with the invertebrate TPPPs. TPPP/p25 associates with microtubules and plays a pivotal role in microtubule dynamics, bundling, and polymerization, thereby stabilizing the microtubular network. This is essential for cytoskeletal organization and proper functioning of neurons and glial cells, including axonal growth, regeneration, migration, trafficking, synapse formation, and myelination of axons. However, studies have also uncovered that besides its cytoplasmic/microtubular localization, TPPP/p25 is present in other subcellular compartments, including the mitochondria and nucleus, underscoring the presence of additional novel functions. At the molecular level, TPPP/p25 is predicted to exist as an intrinsically disordered protein and is implicated in neurological and neurodegenerative disorders, including Parkinson's and related disorders and Multiple Sclerosis. In this article, we provide a comprehensive overview of TPPP/p25, highlighting its evolutionary conservation, cellular and subcellular localization, established and emerging functions in the nervous system, interacting partners, potential clinical relevance to human neurological disorders, and conclude with unresolved questions and future areas of study.
Keywords: CNS; ensheathment; microtubule; mitochondria; α‐synucleinopathies.
© 2025 The Author(s). Journal of Neuroscience Research published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Acevedo, K. , Li R., Soo P., et al. 2007. “The Phosphorylation of p25/TPPP by LIM Kinase 1 Inhibits Its Ability to Assemble Microtubules.” Experimental Cell Research 313: 4091–4106. - PubMed
-
- Alagundagi, D. B. , Ghate S. D., Shetty P., Gollapalli P., Shetty P., and Patil P.. 2023. “Integrated Molecular‐Network Analysis Reveals Infertility‐Associated Key Genes and Transcription Factors in the Non‐Obstructive Azoospermia.” European Journal of Obstetrics, Gynecology, and Reproductive Biology 288: 183–190. - PubMed
-
- Aoki, M. , Segawa H., Naito M., and Okamoto H.. 2014. “Identification of Possible Downstream Genes Required for the Extension of Peripheral Axons in Primary Sensory Neurons.” Biochemical and Biophysical Research Communications 445: 357–362. - PubMed
-
- Birgisdottir, A. B. , Lamark T., and Johansen T.. 2013. “The LIR Motif—Crucial for Selective Autophagy.” Journal of Cell Science 126: 3237–3247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

