Bilateral Focused Ultrasound Thalamotomy for Essential Tremor: Clinical Outcomes Compared to Bilateral Deep Brain Stimulation and Probabilistic Lesion Mapping
- PMID: 40318052
- PMCID: PMC12273620
- DOI: 10.1002/mds.30221
Bilateral Focused Ultrasound Thalamotomy for Essential Tremor: Clinical Outcomes Compared to Bilateral Deep Brain Stimulation and Probabilistic Lesion Mapping
Abstract
Background: The efficacy and adverse events (AEs) of bilateral magnetic resonance-guided focused ultrasound (MRgFUS) thalamotomies for essential tremor (ET) have not been compared to those of deep brain stimulation (DBS). Furthermore, it is uncertain whether second-side thalamotomies can be positioned differently from the first without compromising effectiveness.
Objective: We aimed to indirectly compare bilateral MRgFUS and DBS, while identifying optimal lesion/stimulation locations.
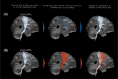
Methods: We retrospectively examined 41 ET patients who received either bilateral thalamic DBS (n = 22) or MRgFUS (n = 19) surgery. The primary outcome was the comparison of modalities for change in Clinical Rating Scale for Tremor (CRST) from baseline to post second surgery. We characterized AEs, generated probabilistic maps, and tracked streamlines intersecting lesions. First-side lesions were always intentionally placed ventrally (z = 0/+2 mm above the intercommissural plane [ICP]), and second-side lesions were placed dorsally (z = +3 mm above ICP).
Results: Tremor scores improved significantly after second surgeries (MRgFUS: 56.3 ± 7.1 to 24.2 ± 10.4, P < 0.001; DBS: 58.8 ± 11.6 to 25.0 ± 13, P < 0.05, mean follow-up: 23/26 months), with no differences between modalities. Following first surgeries, scores were MRgFUS: 37.9 ± 7.9 and DBS: 35.2 ± 13.6, with significant improvement from baseline (P < 0.001, mean follow-up: 40/73 months). All AEs were grade 1-2, with AE-free rates of 41% for DBS and 32% for MRgFUS. First-side lesions exhibited maximal efficacy in the ventral Vim, extending to posterior subthalamic area (PSA), whereas second-side lesions demonstrated maximal efficacy in the dorsomedial Vim-Vop border. DBS maps corroborated this finding and confined to Vim-Vop border. Lesions intersecting with networks interconnected with the supplementary motor area, in addition to M1, were associated with improved outcomes.
Conclusions: The efficacies of bilateral MRgFUS and DBS appear comparable. MRgFUS probabilistic maps vary with different targeting methods, revealing two distinct sweet spots: dorsal Vim-Vop border and ventral Vim/PSA. © 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: bilateral surgery; deep brain stimulation; fiber filtering; focused ultrasound; probabilistic mapping.
© 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures



References
-
- Iorio‐Morin C, Yamamoto K, Sarica C, Zemmar A, Levesque M, Brisebois S, et al. Bilateral focused ultrasound thalamotomy for essential tremor (best‐fus phase 2 trial). Mov Disord 2021;36(11):2653–2662. - PubMed
-
- Fukutome K, Hirabayashi H, Osakada Y, Kuga Y, Ohnishi H. Bilateral magnetic resonance imaging‐guided focused ultrasound thalamotomy for essential tremor. Stereotact Funct Neurosurg 2022;100(1):44–52. - PubMed
-
- Martínez‐Fernández R, Mahendran S, Pineda‐Pardo JA, Imbach LL, Máñez‐Miró JU, Büchele F, et al. Bilateral staged magnetic resonance‐guided focused ultrasound thalamotomy for the treatment of essential tremor: a case series study. J Neurol Neurosurg Psychiatry 2021;92(9):927–931. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

