Defective protein persulfidation is involved in obesity associated skeletal muscle dysfunction: role of SIRT-1
- PMID: 40318302
- PMCID: PMC12124727
- DOI: 10.1016/j.redox.2025.103645
Defective protein persulfidation is involved in obesity associated skeletal muscle dysfunction: role of SIRT-1
Abstract
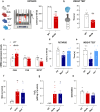
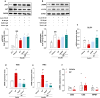
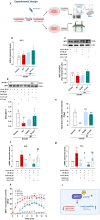
Ectopic fat deposition in skeletal muscle (SKM) due to obesity leads to biochemical and morphological alterations that deteriorate SKM quality and performance. Here, we show that impaired MPST-derived hydrogen sulfide (H2S) signaling contributes to obesity-related SKM dysfunction. Muscle tissues from obese db/db mice exhibit reduced MPST expression, correlating with decreased protein persulfidation and muscle performance in vivo. Mpst-/- mice show similar deficits as db/db mice, confirming the role of MPST. H2S supplementation improves locomotor activity in db/db mice and restores protein persulfidation, including SIRT-1. Myotubes placed in an "obese environment" display a downregulation of MPST, coupled with a reduced SIRT-1 persulfidation leading to an inflammatory state. Exogenous H2S exerts beneficial effects recovering SIRT-1 persulfidation/activity. Finally, muscle biopsies from obese individuals show reduced MPST expression, underscoring the translational relevance to human SKM health. Our study unveils a crucial role for MPST-derived H2S in obesity-associated SKM dysfunction via SIRT-1 persulfidation, highlighting the importance of the MPST/H2S pathway in maintaining healthy SKM function.
Keywords: Db/db mice; H(2)S donors; Persulfidation; Skeletal muscle; Sodium palmitate.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures






References
-
- Vellecco V., Mancini A., Ianaro A., Calderone V., Attanasio C., Cantalupo A., Andria B., Savoia G., Panza E., Di Martino A., Cirino G., Bucci M. Cystathionine β-synthase-derived hydrogen sulfide is involved in human malignant hyperthermia. Clin. Sci. (Lond.) 2016;130:35–44. doi: 10.1042/CS20150521. - DOI - PubMed
-
- Panza E., Vellecco V., Iannotti F.A., Paris D., Manzo O.L., Smimmo M., Mitilini N., Boscaino A., de Dominicis G., Bucci M., Di Lorenzo A., Cirino G. Duchenne's muscular dystrophy involves a defective transsulfuration pathway activity. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102040. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

