Non-canonical Wnt signaling promotes epithelial fluidization in the repairing airway
- PMID: 40319020
- PMCID: PMC12049509
- DOI: 10.1038/s41467-025-59320-1
Non-canonical Wnt signaling promotes epithelial fluidization in the repairing airway
Abstract
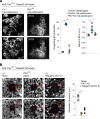
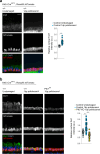
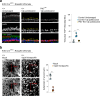
Concerted migration of basal stem cells (BCs) in the airway, also known as epithelial fluidization, has been implicated in epithelial repair after injury. How BC migration is regulated, and how it influences the success of epithelial repair, remains unclear. Here we have identified non-canonical Wnt signaling through Ptk7, Fzd7, and YAP as a critical regulator of BC migration in the mouse trachea. Using live imaging and genetic studies in the mouse, we find that Ptk7 is required for the concerted movement of BCs after injury, and that this requirement extends to BC proliferation and subsequent restoration of epithelial homeostasis after injury. We demonstrate that Ptk7 exerts this function in conjunction with Wnt5a and Fzd7, and through YAP activation in BCs. Our data provide mechanistic insight into the regulation of epithelial repair in the airway.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

