Rare genetic variation in PTPRB is associated with central serous chorioretinopathy, varicose veins and glaucoma
- PMID: 40319023
- PMCID: PMC12049426
- DOI: 10.1038/s41467-025-58686-6
Rare genetic variation in PTPRB is associated with central serous chorioretinopathy, varicose veins and glaucoma
Abstract
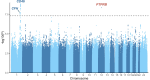
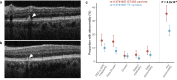
Central serous chorioretinopathy is an eye disease characterized by fluid buildup under the central retina whose etiology is not well understood. Abnormal choroidal veins in central serous chorioretinopathy patients have been shown to have similarities with varicose veins. To identify potential mechanisms, we analyzed genotype data from 1,477 patients and 455,449 controls in FinnGen. We identified an association for a low-frequency (allele frequency = 0.5%) missense variant (rs113791087) in PTPRB, the gene encoding vascular endothelial protein tyrosine phosphatase (odds ratio=2.85, P = 4.5 × 10-9). This was confirmed in a meta-analysis of 2,452 patients and 865,767 controls from 4 studies (odds ratio=3.06, P = 7.4 × 10-15). Rs113791087 was associated with a 56% higher prevalence of retinal abnormalities (35.3% vs 22.6%, P = 8.0 × 10-4) in 708 UK Biobank participants and, surprisingly, with increased risk of varicose veins (odds ratio=1.31, P = 2.3 × 10-11) and reduced risk of glaucoma (odds ratio=0.82, P = 6.9 × 10-9). Predicted loss-of-function variants in PTPRB, though rare in number, were associated with central serous chorioretinopathy in All of Us (odds ratio=17.09, P = 0.018). These findings highlight the significance of vascular endothelial protein tyrosine phosphatase in diverse ocular and systemic veno-vascular diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Dr. Rossin and Dr. Rämö are named inventors on a provisional patent application that describes the secondary use of intravitreal Anti-Ang2 medications for use in the treatment of central serous chorioretinopathy. Dr. Ellinor receives sponsored research support from Bayer AG, IBM Research, Bristol Myers Squibb, Pfizer and Novo Nordisk; he has also served on advisory boards or consulted for MyoKardia and Bayer AG. The remaining authors declare no competing interests.
Figures




Update of
-
Rare genetic variation in VE-PTP is associated with central serous chorioretinopathy, venous dysfunction and glaucoma.medRxiv [Preprint]. 2024 May 9:2024.05.08.24307013. doi: 10.1101/2024.05.08.24307013. medRxiv. 2024. Update in: Nat Commun. 2025 May 3;16(1):4127. doi: 10.1038/s41467-025-58686-6. PMID: 38766240 Free PMC article. Updated. Preprint.
References
-
- Kitzmann, A. S., Pulido, J. S., Diehl, N. N., Hodge, D. O. & Burke, J. P. The Incidence of Central Serous Chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology115, 169–173 (2008). - PubMed
-
- Feenstra H. M. A. et al. Central serous chorioretinopathy: An evidence-based treatment guideline. Prog Retin Eye Res. 101236 (2024). - PubMed
-
- van Rijssen, T. J. et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin Eye Res73, 100770 (2019). - PubMed
-
- Sahoo, N. K. et al. Prevalence and Profile of Central Serous Chorioretinopathy in an Indian Cohort. Nepal J. Ophthalmol.11, 5–10 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 EY035342/EY/NEI NIH HHS/United States
- P30 EY011373/EY/NEI NIH HHS/United States
- OT2 OD026556/OD/NIH HHS/United States
- U2C OD023196/OD/NIH HHS/United States
- OT2 OD025315/OD/NIH HHS/United States
- U24 OD023121/OD/NIH HHS/United States
- OT2 OD026552/OD/NIH HHS/United States
- OT2 OD026549/OD/NIH HHS/United States
- OT2 OD025337/OD/NIH HHS/United States
- P30 EY025585/EY/NEI NIH HHS/United States
- OT2 OD025277/OD/NIH HHS/United States
- OT2 OD026555/OD/NIH HHS/United States
- OT2 OD026550/OD/NIH HHS/United States
- OT2 OD026553/OD/NIH HHS/United States
- I01 BX004557/BX/BLRD VA/United States
- OT2 OD025276/OD/NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- OT2 OD026557/OD/NIH HHS/United States
- OT2 OD026554/OD/NIH HHS/United States
- I01 BX003364/BX/BLRD VA/United States
- OT2 OD023206/OD/NIH HHS/United States
- U24 OD023176/OD/NIH HHS/United States
- 1K23EY035342/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- OT2 OD026548/OD/NIH HHS/United States
- K12 EY016335/EY/NEI NIH HHS/United States
- OT2 OD026551/OD/NIH HHS/United States
- OT2 OD023205/OD/NIH HHS/United States
- IK6 BX005233/BX/BLRD VA/United States
- U24 OD023163/OD/NIH HHS/United States
- K12EY016335/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical

