Latent retinal structural patterns with aging
- PMID: 40319185
- PMCID: PMC12049471
- DOI: 10.1038/s41746-025-01632-z
Latent retinal structural patterns with aging
Abstract
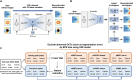
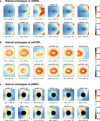
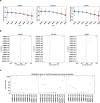
Optical coherence tomography (OCT) is an efficient tool for non-invasively evaluating retinal structures. Retinal thinning changes assessed using OCT are recognized as potential biomarkers for systemic conditions such as Alzheimer's disease, Parkinson's disease, and chronic kidney disease. However, age-related retinal changes remain largely unexplored, complicating the differentiation between physiological and pathological alterations. Here, we introduced a highly granular approach to assess age-related spatial changes in the inner retina using latent retinal archetypes, identifying 36 retinal archetypes of macula and peripapillary sector images from 189,387 OCTs of 22,494 individuals. Subsequently, we evaluated the associations between these archetypes and age; age-related archetypes are characterized by total or superior thinning in the macula sector. Among individuals with myopia, the inferior thinning pattern in the macula ganglion cell-inner plexiform layer was associated with aging. The age-related effects in the peripapillary sector were primarily reflected in the shape of retinal artery trajectories. Overall, latent retinal archetypes would offer new avenues for the effective utilization of retinal biomarkers in age-related diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Xie, J. S., Donaldson, L. & Margolin, E. The use of optical coherence tomography in neurology: a review. Brain145, 4160–4177 (2022). - PubMed
-
- Ito, Y. et al. Quantitative assessment of the retina using oct and associations with cognitive function. Ophthalmology127, 107–118 (2020). - PubMed
-
- Costanzo, E. et al. Ocular biomarkers for alzheimer disease dementia: an umbrella review of systematic reviews and meta-analyses. JAMA Ophthalmol.141, 84–91 (2023). - PubMed
-
- Satue, M. et al. Retinal thinning and correlation with functional disability in patients with Parkinson’s disease. Br. J. Ophthalmol.98, 350–355 (2014). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

